Guerbet is highlighting that the European Commission has approved the marketing of Elucirem (gadopiclenol).
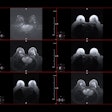
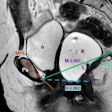
Elucirem is a macrocyclic gadolinium-based contrast agent (GBCA) for use in contrast-enhanced MRI. The company said it has high relaxivity and is indicated for adults and children aged 2 and older to improve detection and visualization of pathologies with disruption of the blood-brain barrier. This also includes abnormal vascularity of the brain, spine, and associated tissues of the central nervous system, as well as the liver, kidney, pancreas, breast, lung, prostate, and musculoskeletal system.
Guerbet said that the European Commission is the second regulatory organization to approve Elucirem, with further evaluation continuing in other countries.
Gadopiclenol is sold under the trade names Vueway and Elucirem by Bracco and Guerbet respectively.