Pediatric diagnostic reference levels (DRLs) may be more effectively established for fluoroscopic procedures using a dose-age curve method than using conventional methods, according to findings from a study published in European Radiology on 6 September.
The aim of the study, led by medical physicist Goswin Croes of Isala Hospital in Zwolle and Radboud University Medical Center in Nijmegen, was to assess pediatric fluoroscopic dose levels in clinical practice for micturating cystourethrography (MCU), upper gastrointestinal (GI), and lower GI procedures to update Dutch pediatric DRLs to correspond with the recommendations of the European Guidelines on DRLs for Pediatric Imaging (PiDRL).
Additionally, the team sought to compare the levels assessed using the conventional method based on the kerma-area product (KAP) values determined per discrete weight/age group as defined in the PiDRL guidelines, and a curve method developed for sparse data based on the local DRL curves of each hospital.
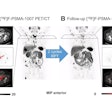
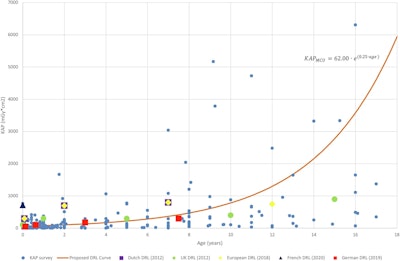 Diagnostic reference level (DRL) curve method for micturating cystourethrography (MCU), showing air kerma-area product (KAP) values from surveyed hospitals and the proposed DRL curve (including equation) alongside Dutch, U.K., European, French, and German DRLs.Courtesy Croes et al., European Radiology. Image available for republishing under Creative Commons license (CC BY-NC-ND 4.0).
Diagnostic reference level (DRL) curve method for micturating cystourethrography (MCU), showing air kerma-area product (KAP) values from surveyed hospitals and the proposed DRL curve (including equation) alongside Dutch, U.K., European, French, and German DRLs.Courtesy Croes et al., European Radiology. Image available for republishing under Creative Commons license (CC BY-NC-ND 4.0).
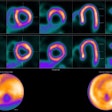
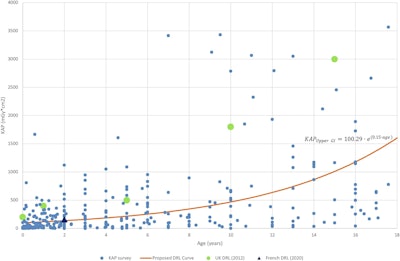 Diagnostic reference level (DRL) curve method for upper GI, showing air KAP values from surveyed hospitals, and the proposed DRL curve (including equation) compared with U.K. and French DRLs.Courtesy Croes et al; European Radiology
Diagnostic reference level (DRL) curve method for upper GI, showing air KAP values from surveyed hospitals, and the proposed DRL curve (including equation) compared with U.K. and French DRLs.Courtesy Croes et al; European Radiology
Developing DRLs for pediatric patients is challenging due to a large range of variation in size and weight among different age groups and the paucity of pediatric dose data. The authors explained that the proposed curve method establishes DRL dose-weight or dose-age curves -- this is in contrast to the conventional approach, in which a DRL is established for each age group. The new method allows pediatric fluoroscopy procedures “to be compared to the DRL at any age,” they wrote.
Study logistics
The study used retrospective data on three pediatric (birth to 18 years) fluoroscopic procedures (MCU, upper GI, lower GI) from a national survey conducted between April and June 2021 by the Dutch PiDRL working group with support from the Dutch Radiological Society (NVvR), the Dutch Society of Medical Imaging and Radiotherapy (NVMBR), and the Dutch Medical Physics Society (NVKF). Dose data were collected from seven Dutch hospitals (4 academic and 3 general) that participated in the survey, with a total of 971 examinations.
For comparison, KAP values were retrospectively collected from pediatric patients who underwent fluoroscopic exams between 1 January 2017 and 6 January 2021. This is the conventional approach for establishing DRLs recommended by the European PiDRL guidelines; it was referred to as “Method 1” in the study.
“According to these guidelines, only data from hospitals that provided data of more than 20 examinations for a given fluoroscopy protocol in each age group (0 < 1 month; 1 month < 4 years; 4 < 10 years; 10 < 14 years; 14 < 18 years) were used. The median (Q2) of the KAP was determined for each fluoroscopy protocol per age group per hospital. The DRL for dose was then calculated as the 75th percentile (Q3) of the medians of all hospitals per age group.”
For the dose-age method, referred to as Method 2 in the study, a quantile regression was applied to the dose-age data of each hospital separately to estimate its 50th percentile dose curve. After values were derived from the separate 50th percentile dose curves per hospital, quantile regression was applied to the newly constructed dataset using the discrete data points of all the hospitals to generate a 75th percentile curve representing the DRL curve.
The results produced by Method 2 were then compared to available Dutch and other available national European DRLs; additionally, the MCU DRL was compared with the existing Dutch DRL. A comparison was also made for all protocols to European, U.K., French, and German DRLs as well.
Of note is that the DRL of the MCU could not be calculated for age groups ≥ 10 years for Method 1, as the data available consisted of fewer than 20 examinations per hospital. However, this was not an issue for Method 2. Only two hospitals provided data for lower GI; therefore, the DRL for this procedure was not calculated for either method. There was adequate data for upper GI to determine DRL for both methods, the authors added.
In the comparison with other national DRLs, the DRL curve values for upper GI were lower than those of the U.K. DRLs. For the MCU results, the DRL curve values were substantially lower than the current Dutch DRLs, confirming the need for updated Dutch DRLs for MCU (the European guidelines suggest updates every five years; the current DRLs were established in 2013).
As the dose-age method allows for calculating DRLs from smaller data sets, as with the MCU results, it may prove more accessible as a method, the authors write. Additionally, the weight of patients -- an important element for DRL calculation in pediatrics -- is not always available. However, age range is generally correlated with size; age “can be easily found in the electronic medical record and dose management system,” the authors noted.
“By choosing an age-based curve method, the assessment becomes relatively easy to implement for hospitals,” they concluded.
Read the full study here.