While FDG-PET has become the standard for tracking patients’ neurodegeneration patterns, early perfusion imaging added to amyloid-PET has been investigated as a promising option, providing the additional ability to track amyloidosis.
In an article earmarked for the December 2025 edition of the European Association of Nuclear Medicine (EANM) Journal, a research group headed by Prof. Silvia Morbelli, PhD, from the Nuclear Medicine Unit at the University of Turin in Italy, discussed the position of the EANM on early perfusion imaging used as part of a dual-phase protocol in amyloid-PET studies.
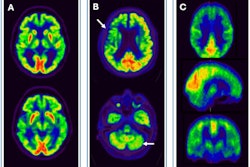
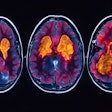
![FDG, early perfusion amyloid-PET, and late standard amyloid-PET in a patient with Alzheimer’s disease (AD) ([F-18] Flutemetamol, panel A) and in a patient with behavioral variant of fronto-temporal dementia (bvFTD) ([F-18] Florbetaben, panel B). Regardless of the disease-related pattern (posterior in patient A and anterior in patient B), FDG and early perfusion imaging show largely overlapping distribution with small differences. Higher early-phase amyloid tracer distribution with respect to FDG uptake is expected in the brain stem, cerebellum, anterior cingulate, and thalamus, while lower uptake compared to FDG is present in the prefrontal, orbitofrontal, posterior parietal, and superior temporal regions.](https://img.auntminnieeurope.com/mindful/smg/workspaces/default/uploads/2025/10/2025-10-20-ame-fig1.P05I0aTgvZ.jpg?auto=format%2Ccompress&fit=max&q=70&w=400) FDG, early perfusion amyloid-PET, and late standard amyloid-PET in a patient with Alzheimer’s disease (AD) ([F-18] Flutemetamol, panel A) and in a patient with behavioral variant of fronto-temporal dementia (bvFTD) ([F-18] Florbetaben, panel B). Regardless of the disease-related pattern (posterior in patient A and anterior in patient B), FDG and early perfusion imaging show largely overlapping distribution with small differences. Higher early-phase amyloid tracer distribution with respect to FDG uptake is expected in the brain stem, cerebellum, anterior cingulate, and thalamus, while lower uptake compared to FDG is present in the prefrontal, orbitofrontal, posterior parietal, and superior temporal regions.Prof. Silvia Morbelli et al and EANM Journal
FDG, early perfusion amyloid-PET, and late standard amyloid-PET in a patient with Alzheimer’s disease (AD) ([F-18] Flutemetamol, panel A) and in a patient with behavioral variant of fronto-temporal dementia (bvFTD) ([F-18] Florbetaben, panel B). Regardless of the disease-related pattern (posterior in patient A and anterior in patient B), FDG and early perfusion imaging show largely overlapping distribution with small differences. Higher early-phase amyloid tracer distribution with respect to FDG uptake is expected in the brain stem, cerebellum, anterior cingulate, and thalamus, while lower uptake compared to FDG is present in the prefrontal, orbitofrontal, posterior parietal, and superior temporal regions.Prof. Silvia Morbelli et al and EANM Journal
The authors acknowledge that early perfusion amyloid-PET as a dual-point protocol -- as both procedures can be performed sequentially on the same day -- shows potential for diagnosing and monitoring neurodegenerative disease using the AT(N) biomarker system, especially in complex cases.
The AT(N) (beta-amyloid, tau, neurodegeneration) system defines and stages Alzheimer’s disease and neurodegenerative dementia in patients based on fluid/imaging biomarkers. While imaging biomarkers can provide topographical details, currently, each process (i.e., A, T, N) must be tracked individually: amyloid-PET is used for A (beta-amyloid plaques); tau PET is used for cortical and subcortical tau deposits (T); and [F-18] fluoro-2-deoxyglucose (FDG)-PET to track neurodegeneration.
The researchers noted that the AT(N) approach may entail multiple tests depending on the suspected disease. For example, many patients who need amyloid-PET imaging for suspected Alzheimer’s disease may also need to be evaluated for neurodegeneration using FDG-PET, the standard radiopharmaceutical used for identifying neurodegenerative disease-related patterns.
Studies show that early-perfusion imaging could have potential as a surrogate for FDG, they explained. Although it is still at the research-only stage, recent study findings have shown that early perfusion amyloid-PET imaging can categorize both amyloidosis (A) and neurodegeneration (N) biomarkers, potentially offering “a practical opportunity to obtain A and N categories with a noninvasive procedure in the clinical setting.”
Early-perfusion imaging may be performed on the same day as standard amyloid PET, providing complementary data in what they refer to as a “one-stop shop” approach, especially in more complex pathologies and those involving comorbidities, the authors pointed out.
Furthermore, perfusion images can aid in improving the quantification of amyloid-PET imaging. It can be impaired by many factors, including proper identification of the reference region, changes in brain perfusion, both in target and reference regions, or partial volume effects, they added.
While strategies to mitigate factors that may impair the estimation of semiquantitative values obtained from static amyloid-PET studies have been proposed, they noted that there is “still a lack of software providing a dedicated pipeline.” However, “the definition of integrated semiquantification pipelines, the availability of dedicated templates and normative datasets represent gating milestones for a clinical implementation of early-phase amyloid PET.”
In addition, the requirement of two separate exams on two days (as with FDG-PET) is less economical in terms of money, resources, and time. The authors wrote that not the time saved is a significant advantage for patients, staff, and caregivers: “The latter aspect is not to be underestimated in the target cohort, namely patients with dementia, who often show limited capabilities to cooperate.”
There are instances where the use of the amyloid-PET dual-phase protocol might be specifically better than FDG-PET. The researchers give the example of patients with uncontrolled diabetes or complex medication schedules who cannot fast, as fasting is not necessary for early-phase amyloid PET and there is no interference with the patient’s glucose levels.
Extrapolating to other imaging, the authors suggest that such a dual-phase protocol could also be applicable to other PET radiotracers; in particular, they write that a dual-phase approach for tau-PET could yield similar results and benefits for tau (T) and neuronal neurodegeneration (N) for Parkinsonian syndrome.
The authors conclude that the addition of early perfusion imaging to amyloid-PET acquisition in a dual-phase approach shows promise in replacing FDG as a separate procedure when both the amyloid and neurodegenerative data are important for diagnosis. There is currently a lack of clinical trial data, especially in mild cognitive impairment (MCI) patients, they noted.
"While early perfusion imaging is, currently, still a research tool, this technique should be further considered in future updates of the AT(N) and other Alzheimer's disease biomarker classification concepts, defining the population for which this adapted protocol could become standard," the researchers wrote.
In clinical care, the question of reimbursement for providing the additional “N” biomarker out of only one amyloid tracer injection is relevant, they stated. "In this regard, a complete translation to clinical practice of amyloid-PET dual-point protocol requires not only prospective clinical trials but also more general support by the PET imaging community."
Read the article here. The co-authors were Donatienne Van Weehaeghe, Antoine Verger, Nelleke Tolboom, Pablo Aguiar Fernandez, Matthias Brendel, Eric Guedj, Valentina Garibotto, Diego Cecchin, Igor Yakushev, Bart van Berckel, Nathalie L. Albert, Francesco Fraioli, Tatjana Traub-Weidinger, and Henryk Barthel.