Dynamic contrast-enhanced (DCE) MRI plus measuring changes in the pharmacokinetic extracellular space are useful tools for predicting the response of brain metastases in lung cancer patients, French researchers reported in a study published online on 16 February in European Radiology.
Lung cancer patients with brain metastases prospectively underwent conventional 3-tesla MRI at baseline, during treatment, and after treatment. DCE-MRI was performed at baseline and early post-treatment to evaluate certain pharmacokinetic parameters. The research team, led by Dr. Grégory Kuchcinski from the neuroradiology department at the University of Lille, in Lille, France, found the combination of DCE-MRI plus change in extravascular extracellular space per unit volume of tissue to be the best response predictor.
"The early variation of [extravascular extracellular space per unit volume of tissue] (seven to 10 weeks post-treatment) was the best predictor of response at midterm follow-up," he and his colleagues wrote. "DCE-MRI enables a quantitative assessment of the extravascular extracellular space expansion within each voxel through the pharmacokinetic parameter extravascular extracellular space per unit volume of tissue, which is elevated in brain metastases, glioblastomas, and various extracranial tumors."
Why DCE-MRI?
Brain metastases occur in 10% to 20% of patients with localized lung cancer, which can affect quality of life and overall survival. The therapeutic strategy is usually local treatments such as surgery, radiosurgery, or whole-brain radiotherapy, and it depends on the number, localization, and size of brain metastases; control of extra-central nervous system disease; and the general condition of patients, according to the researchers.
Current guidelines to assess brain metastases are based on size variation on conventional MRI, but radiological enlargement during the early period after treatment does not necessarily represent tumor progression, especially in patients treated by radiosurgery or targeted therapy.
"Given the potential benefits of second-line therapies, it is crucial to determine predictors of response during the early follow-up," Kuchcinski and colleagues wrote. "Previous studies have demonstrated the increasing abilities of advanced MRI techniques to predict the response in patients with brain metastases."
DCE-MRI enables the assessment of the blood-brain barrier (BBB) integrity and neoangiogenesis within brain tumors by tracking changes in a gadolinium contrast agent. The researchers hypothesized that increased BBB permeability and neoangiogenesis in brain metastases were associated with a better response to antineoplastic therapy and the early variation of the DCE-MRI parameters could be predictive of the objective response.
They included 44 consecutive lung cancer patients with 123 newly diagnosed brain metastases. Conventional MRI (Achieva, Philips Healthcare) was conducted at baseline (within one month before treatment) and during the early (seven to 10 weeks) and midterm (five to seven months) periods after treatment.
An additional DCE-MRI sequence was performed during baseline and early post-treatment MRI to evaluate baseline pharmacokinetic parameters and their early variation. The objective response was evaluated by the volume variation of each metastasis from baseline to midterm MRI. Receiver operating characteristic curve analysis determined the best DCE-MRI parameter to predict the objective response.
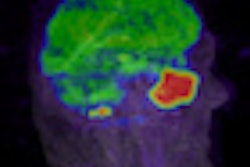
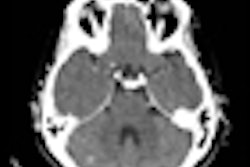
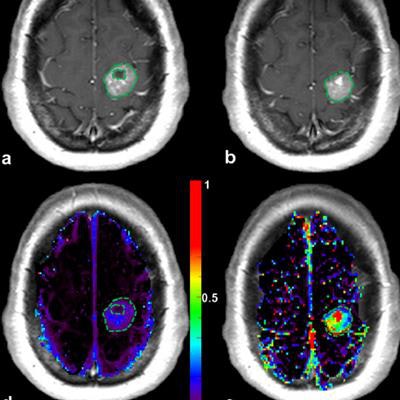
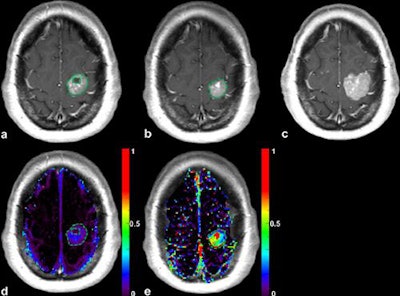 Patient with a left precentral metastasis on T1-weighted images at baseline (a), early follow-up (b), midterm follow-up (c), parametric map of extravascular extracellular space per unit volume of tissue at baseline (d), and midterm follow-up (e). A volumetric regression of the lesion was observed on the conventional T1-weighted sequence with gadolinium injection between baseline (a) and early follow-up (b). DCE-MRI parametric map demonstrated an increase of the extravascular extracellular space per unit volume of tissue value between baseline (d) and early follow-up (e), which predicted the volumetric progression of the lesion at midterm follow-up (c). The green dotted line represents the region of interest used for DCE-MRI measurement at baseline (a, d) and early follow-up (b, e), corresponding to the enhancing part of the lesion at the level of maximum section area with careful exclusion of necrotic components. Image courtesy of Dr. Grégory Kuchcinski and European Radiology.
Patient with a left precentral metastasis on T1-weighted images at baseline (a), early follow-up (b), midterm follow-up (c), parametric map of extravascular extracellular space per unit volume of tissue at baseline (d), and midterm follow-up (e). A volumetric regression of the lesion was observed on the conventional T1-weighted sequence with gadolinium injection between baseline (a) and early follow-up (b). DCE-MRI parametric map demonstrated an increase of the extravascular extracellular space per unit volume of tissue value between baseline (d) and early follow-up (e), which predicted the volumetric progression of the lesion at midterm follow-up (c). The green dotted line represents the region of interest used for DCE-MRI measurement at baseline (a, d) and early follow-up (b, e), corresponding to the enhancing part of the lesion at the level of maximum section area with careful exclusion of necrotic components. Image courtesy of Dr. Grégory Kuchcinski and European Radiology.The best objective response
Baseline DCE-MRI parameters were not associated with objective response, Kuchcinski and colleagues found. However, objective response was associated with early change in transfer content, extravascular extracellular space per unit volume of tissue, and plasmatic space per unit volume of tissue (p = 0.02, p = 0.001, and p = 0.02, respectively).
The best predictor of objective response was change in extravascular extracellular space per unit volume of tissue, with an area under the curve of 0.93.
"In our study, the early MRI evaluation was performed seven to 10 weeks after treatment, which is in agreement with the routine follow-up of patients with brain metastases and therefore practical in a clinical setting," the researchers wrote. "Nevertheless, the optimal timing of the early MRI evaluation remains to be determined."
They also noted that given the potential benefits of second-line treatments, metastases with a stable or increased extravascular extracellular space per unit volume of tissue (i.e., > -12.5%) during the early post-treatment period would benefit from short-interval MRI follow-up to anticipate the diagnosis of tumor progression.
In terms of patients with multiple brain metastases and heterogeneous response, the results of this study suggest the risk of global nonresponse is higher in patients with at least one metastasis with change in extravascular extracellular space per unit volume of tissue > -12.5%.
The researchers acknowledged the size of their cohort is a limitation and more studies are needed. Nevertheless, their preliminary results show the potential value of DCE-MRI and extravascular extracellular space per unit volume of tissue as a biomarker of the response of brain metastases from lung cancer to antineoplastic therapy.
The early variation of the biomarker may "help the clinician to identify patients with high risk of progression, to individualize patient's oncological follow-up, and anticipate second-line treatments," they concluded.