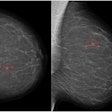
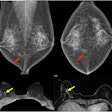
Vara has received a CE certificate for its new AI breast-imaging software, marking the company’s entry into the European market.
The new CE-marked product will be available in Europe on 15 October. The software has been approved as an independent second reader for screening and diagnostic use as a decision support system, including for analysis of mammograms from prior screening rounds, the company noted.
Providers can integrate the software directly into their PACS viewer or via the Vara platform, which supports 40% of screening centers in the German national screening program, processing about 150,000 mammograms monthly and more than 1.5 million annually, the vendor noted.
The platform has been validated through the PRAIM (PRospective multicenter observational study of an integrated AI system with live Monitoring) study, a prospective multicenter study that enrolled more than 460,000 women in 12 centers across Germany, Vara pointed out. Findings of the PRAIM study were published in Nature Medicine earlier this year.