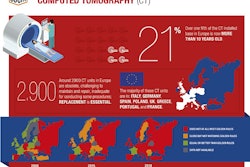
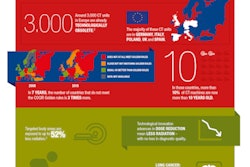
The European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry (COCIR) has published a position paper that offers a strategic and in-depth overview of the impact of the Medical Device Regulation (MDR) framework.
The report outlines COCIR's views on the general framework, premarket and postmarket obligations for manufacturers, and the relevant actors in the MDR implementation process, according to the organization. It will be updated regularly throughout the year.
"COCIR continues to be highly supportive of the European Commission and the member states, and is ready to continue its concrete support toward successful MDR implementation as it is crucial for our industries to avoid unpredictable delays on time-to-market, especially as our industries are highly innovative," said COCIR Secretary General Nicole Denjoy in a statement. These innovations ultimately benefit patients and citizens in Europe and beyond, she said.
COCIR said it also acknowledged the European Commission's recent communication on the impact of Brexit, and welcomed the publication of the Competent Authorities for Medical Devices (CAMD) transitional frequently asked questions (FAQ) on 17 January. The document clarifies several questions on the implementation provisions of the MDR, according to the organization.