Blue Earth Diagnostics has received $27.9 million (25.4 million euros) in capital from U.K. venture fund Syncona Partners.
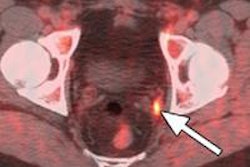
The cash will allow the company to continue its regulatory approval preparations for F-18 fluciclovine, its investigational PET radiopharmaceutical for prostate and brain cancer. The company expects to submit applications to the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for use of the agent in prostate cancer patients experiencing biochemical recurrence.
Blue Earth Diagnostics has signed an agreement with Siemens Healthcare subsidiary PETNet Solutions to manufacture, distribute, and sell the agent in the U.S. once it has been approved, the company said.