The program will cover the European Union Good Distribution Practice requirements of the responsible person (RP) and the role of the RP in the management of the returned products, the change control process, and in batch release.
Routine Duties of the Responsible Person
Oct 26th, 2014
Latest in Home
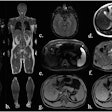
German group shows how to optimize whole-body MRI
October 29, 2025
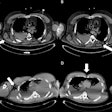
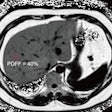
Team Sicily shines light on imaging of gunshot wounds
October 28, 2025
New procedure treats chronic pain in toe joints
October 28, 2025