Hydrophilic polymer embolization (HPE) is a complication of endovascular interventions that tends to be underdiagnosed and under-represented in the literature, European interventional radiology researchers have warned.
HPE is caused by the hydrophilic coating on vascular devices delaminating and being released into the patient’s bloodstream, where it eventually causes distal ischemia or inflammation in different organs or systems, potentially resulting in serious and even fatal outcomes, explained first author Dr. Marco Parillo, from the Department of Radiology, Multizonal Unit of Rovereto and Arco, APSS Trento, Italy, and colleagues.
In an article posted on 20 June by Cardiovascular Interventional Radiology, the authors presented their findings from an analysis of HPE-related events. Their aim was to identify the risks associated with HPE and its clinical implications, any gaps in research, and potential justification for future research.
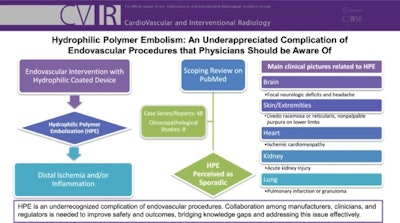 Flowchart explaining HPE in endovascular procedures.Courtesy of Dr. Marco Parillo et al, Cardiovascular and Interventional Radiology
Flowchart explaining HPE in endovascular procedures.Courtesy of Dr. Marco Parillo et al, Cardiovascular and Interventional Radiology
For their overview, the researchers performed a comprehensive search using the PubMed database, encompassing all indexed articles. Their selection criteria targeted research articles and case series or reports in clinical settings. Reviews and overviews were excluded, as the authors wrote that they aimed to include only original studies, and only articles in English were included. A total of 56 reports were ultimately selected for analysis, comprising 48 case series and case reports and eight clinicopathological studies.
The adverse events documented in the literature ranged from asymptomatic to severe, manifesting within days to weeks after the suspected HPE. The serious outcomes included stroke, pulmonary infarction, persistent gangrene, and death.
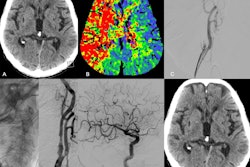
A complicating factor in identifying HPE is that polymer coating emboli within the vasculature often go undetected during standard angiographic procedures due to their typically minute size. The detection of polymer particles therefore relies on histology, the authors wrote, an important aspect of the clinicopathological studies.
In one such study involving a cohort of 136 hospital autopsies, the original study authors found HPE in 18 (13%) of the 136 cases. However, evidence of HPE was documented in only three (17%) of those 18 cases in the autopsy reports; HPE was missed in 83% of the patients in which it was present, the authors wrote.
In another included study, polymer coating deposition was present in 33% of the cerebral thrombectomy specimens analyzed. Furthermore, there was no specific thrombectomy device consistently associated with intrathrombic polymer deposition. The authors noted that the diverse range of devices used during the procedures suggested that the polymer may have come from multiple sources.
For the case studies, while the researchers noted that HPE may affect any part or system of the body and in rare cases may result in multisystem involvement, the analysis showed that HPE was primarily documented in the brain, the skin and extremities, and the heart; it was also documented in the lungs and kidneys with less frequency.
While the effects and symptoms varied by the organ or systems affected, the authors noted that “Polymer deposits, whether located at the access site or embolized to distant or end organs, have been observed to cause vaso-occlusion, leading to peri- and/or intravascular inflammation, fibrosis, and/or thrombosis.” They also note that the early stages of HPE tend to be asymptomatic; when symptoms manifest, they vary by organ, and some may be nonspecific. Furthermore, they may occur months or even years after the endovascular procedure.
The authors of the overview underscore that the case reports, which make up the bulk of the literature on HPE, emphasize the episodic nature of these events. However, with the number of endovascular procedures on the rise, the authors contend that with the “potentially subtle nature of diagnosis” and lack of awareness of the condition, HPE is underreported in the literature.
Additionally, the authors suggested that contributing factors for the occurrence of HPE likely include the size of the instruments being used, repeated or challenging procedures, and longer procedure times. All of these increase the risk of mechanical scraping and peeling of the coating or chemical degradation due to extended exposure to blood or saline.
Thus, along with greater awareness of the condition needed by clinicians, the researchers also suggest practical measures to be taken by manufacturers: the development of polymer coatings with better bonding strength to the device, more rigorous durability testing, comprehensive training programs for clinicians on the devices that take into account the risk of HPE.
They call on clinicians, manufacturers, and regulatory agencies to collaborate on preventing HPE, as well as suggesting that future research may lead to new treatments for managing the effects of HPE.
Read the study here.