Bayer has released updated phase III subgroup study results for its investigational low-dose gadolinium-based contrast agent (GBCA) gadoquatrane in cardiac MRI.
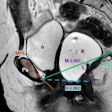
The analysis is based on a subgroup of patients in the company’s phase III study QUANTI OBR (other body regions), which evaluated the efficacy and safety of gadoquatrane in adults with known or suspected pathologies, including cardiac lesions, undergoing contrast-enhanced MRI.
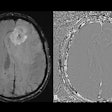
Results showed that gadoquatrane demonstrated similar efficacy and safety to comparator GBCAs while enabling a 60% reduction in administered gadolinium dose, Bayer said. When compared to cardiac MRI scans without contrast enhancement, gadoquatrane demonstrated clear improvement in the subgroup analysis. The findings are in line with results from the overall analysis of Quanti OBR, according to the firm.
Based on positive data from the studies, Bayer has submitted applications for marketing authorization of gadoquatrane in markets around the world, including Japan, the U.S., the European Union, and China, the company noted.