Breast MRI exams using gadopiclenol with a half standard gadolinium dose offer equivalent to superior contrast enhancement and diagnostic confidence compared to gadobutrol-enhanced MRI with full standard gadolinium dose, researchers have reported.
The results are particularly good news for patients who require repeated MR imaging, wrote a team led by Dr. Philipp Gruschwitz, of University Hospital Wuerzburg in Germany. The findings were published on 5 February in Investigative Radiology.
"Reducing cumulative gadolinium exposure is an important clinical objective given concerns about gadolinium deposition in body tissues above all, the brain, even if the clinical relevance is still unclear," the group noted.
Although previous research regarding gadolinium-based contrast agents (GBCAs) suggests they are safe to use, "concerns persist regarding the long-term deposition of gadolinium in body tissues and potential environmental contamination through wastewater effluents," the investigators explained, noting that "this increased deposition is especially concerning for patients with compromised kidney function, as their ability to eliminate gadolinium is reduced, potentially increasing the risk of developing nephrogenic systemic fibrosis."
To address the problem, clinicians and researchers have attempted to boost the use of macrocyclic agents such as gadopiclenol.
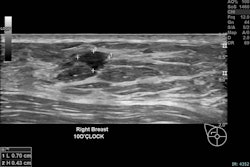
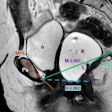
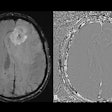
Gruschwitz and colleagues conducted a study that included 145 women who underwent breast MRI with a half-standard dose of gadopiclenol (0.05 mmol/kg) between January and March 2025. They compared these results to a cohort of women who had undergone breast MRI with a standard dose of gadobutrol (0.1 mmol/kg) within two years prior to the study set, assessing signal enhancement of the aorta, axillary lymph nodes, and breast parenchyma. Two radiologist readers evaluated image quality and diagnostic confidence using a 5-point Likert Scale for the two types of exams.
The group reported the following:
Comparison of contrast agents used with breast MRI | |||
Measure | Gadobutrol (0.1 mmol/kg) | Gadopiclenol (0.05 mmol/kg) | p-value |
| Absolute enhancement values (Hounsfield Units) for all regions | 33.4 HU to 56.3 HU | 41.8 HU to 65.4 HU | < 0.001 |
| Overall diagnostic confidence ratings (Likert scale) | 5 | 5 | -- |
| Interreader agreement | Cohen's kappa coefficient = 0.48 | Cohen's kappa coefficient = 0.59 | -- |
| "Excellent" ratings | 56.8% | 71.9% | 0.002 |
The investigators also found that the radiologist readers considered most exam pairs equal in terms of image quality (radiologist 1: 90 out of 145; radiologist 2: 92 out of 145 [p = 0.845]) and that the exams showed fair agreement (Cohen's kappa coefficient = 0.366).
Finally, the team reported that the two readers reached agreement in 97 of the 145 cases (with 67 categorized as "equal," 21 as "pro-gadopiclenol," and 9 as "pro-gadobutrol").
The authors also highlighted that the study findings indicate that using gadopiclenol at half dose of gadobutrol for breast MRI is not only good for patients, but also for the environment.
"The environmental impact [of gadolinium imaging] is reduced by using half standard dose in gadopiclenol scans instead of full standard gadolinium dose of other macrocyclic gadolinium contrast agents, as only half the amount of gadolinium needs to be administered for equivalent imaging," they concluded.
Access the full article here.