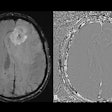
The European Commission (EC) has granted marketing authorization in the European Union for Bracco's Vueway (gadopiclenol).
Vueway is for use with contrast-enhanced MRI in adult patients and children aged two years and older to improve detection and visualization of pathologies with disruption of the blood-brain barrier and/or abnormal vascularity of the brain, spine, and associated tissues of the central nervous system, Bracco said. It was approved by the U.S. Food and Drug Administration (FDA) in September of 2022.
Gadopiclenol is sold under the trade names Vueway and Elucirem by Bracco and Guerbet respectively.