Researchers at the University of Massachusetts Medical School in the U.S. and Tianjin Medical University in China are reporting on the first-ever human protein-based tumor-targeting MRI contrast agent. The biohybrid composite, which can also be easily cleared by the body, could be used to detect tumors in their early stages, thanks to its enhanced MRI contrast and shorter proton relaxation time (Nano Letters, 5 June 2017).
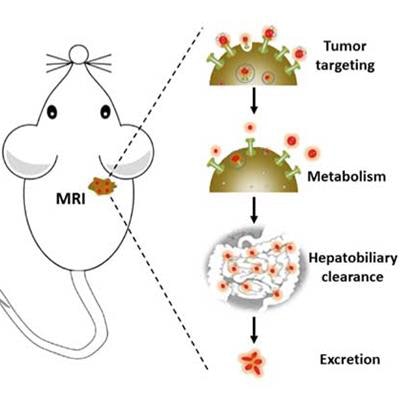
 Gd nanoparticles biomineralized in transferrin proteins. After being injected into the tail vein of mice, Gd@Tf NPs accumulate in the tumor areas and are eliminated by the body via the hepatobiliary system. This shows that Gd@Tf NPs are tumor-targeting and metabolically clearable, say the researchers. Image courtesy of Nano Letters.
Gd nanoparticles biomineralized in transferrin proteins. After being injected into the tail vein of mice, Gd@Tf NPs accumulate in the tumor areas and are eliminated by the body via the hepatobiliary system. This shows that Gd@Tf NPs are tumor-targeting and metabolically clearable, say the researchers. Image courtesy of Nano Letters.MRI is a very good way of observing anatomical structures in soft biological tissue without the need for ionizing radiation or dangerous radionuclides. MR images are obtained by exciting the protons found in water and fat inside the body with a magnetic field and then measuring the rate that these protons relax back to their original state. These proton relaxation times are different for different tissues, which results in the MR contrast in the final image.
The most frequently employed contrast agents in the clinic today are gadolinium-based chelates because they shorten the relaxation time of protons. The proton relaxation itself can be described by a parameter called relaxivity, or contrast enhancement efficiency, and defines how bright the contrast will appear in an MR image. Gd chelates have the added advantage that they do not provoke an immune response in cells -- but they are unfortunately retained in vital organs, which is a problem.
In the search for alternative chelates, researchers have turned their attention to proteins, which are naturally occurring nanomaterials. For example, the albumin-bound paclitaxel nanoparticle Abraxane can be used to treat metastatic breast cancer. In the same vein, protein scaffolds that encapsulate metal-based nanoparticulate contrast agents also appear to enhance the relaxivity of these agents.
A team led by Gang Han is now reporting on transferrin (Tf) protein Gd-based MRI contrast agents prepared by mimicking the natural biomineralization process. Tf protein is an endogenous glycoprotein that plays an important role in metabolizing iron in the body. The Gd@TfNPs, as they are called, are better than conventionally employed Gd chelates because they amplify the MR signal more strongly, have a higher relaxivity, and are very stable. They are also biocompatible, precisely target tumors, and are effectively cleared by the body through the hepatobiliary system.
Gentle approach
Han and colleagues began by dissolving Gd(NO3)3 and Tf in pure water at 37° C. Tf has multiple carboxyl and thiol groups and induces the formation of a Tf-Gd3+ complex. Sodium hydroxide was then added to the mixture to allow Gd2O3 to form and simultaneously unfold Tf, something that precipitates out the Gd2O3 nanoparticles into Tf. After purifying the ensemble, the researchers obtained Gd@TfNPs as a clean, transparent and stable solution."This one-step approach is easy, environmentally benign and reproducible," says Han. "It is also very gentle since it does not alter the physiological characteristics of the Tf template.
Good for visualizing tumor therapies
"The Gd@TfNPs could be used as tumor targeting and systematically clearable contrast agents for MR detection of early-stage tumors," he tells our sister site nanotechweb.org. "The tumor shuttle-like feature of Tf in such nanoparticles might also allow the prepared composites to be employed as an MRI-guided delivery vehicle for a wide range of theranostic applications."Such probes can immediately leave the tumor cell sites after delivery and we could track the overall process by MRI. Such a technique might be useful, not only for visualizing tumor therapies but also optimizing drug dose and evaluating clinical results," adds the first author of the study Yang Zhao.
Belle Dumé is contributing editor at nanotechweb.org.
© IOP Publishing Limited. Republished with permission from nanotechweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.