Delivering safe medical devices. This seminar has been designed to provide a comprehensive overview into the use of ISO 13485 as the basis for a Quality Management System (QMS) for medical device manufacturers.
ISO 13485 Quality Management Systems for Medical Devices
Oct 5th, 2015Oct 6th, 2015
London, --
GB
Phone:+44 (0) 1473 730071
Latest in Home
MRI safety panel unveils findings on patient death
February 13, 2026
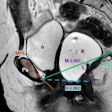
Abdominal pain? Consider fishbones
February 13, 2026
Christiane Kuhl endorses DRG's new women’s health campaign
February 13, 2026
Do young athletes with low back pain need advanced imaging?
February 12, 2026