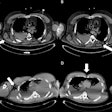
The seminar is especially designed for the MR safety technical interests of auditors and quality/regulatory affairs managers, product and sales managers, R&D managers/engineers, MR clinicians, and MR physicists.
The seminar language is English.
Medical Device Safety Specialist
Oct 9th, 2013Oct 11th, 2013
Latest in Home
Team Sicily shines light on imaging of gunshot wounds
October 28, 2025
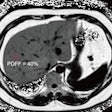
MRI predicts diabetes in obese patients
October 28, 2025