Europe's Committee for Medicinal Products for Human Use (CHMP) has recommended approval of Bracco's MRI imaging agent gadopiclenol solution.
Gadopiclenol (Vueway) was approved in the U.S. in September 2022, with the European Commission's final decision on approval expected by the end of this year, the company said.
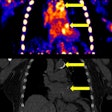
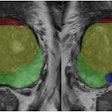
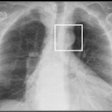
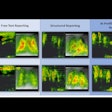
Vueway is a new macrocyclic gadolinium-based contrast agent (GBCA). The CHMP opinion is based on safety and efficacy data from two phase III clinical trials that demonstrated comparable lesion visualization using gadopiclenol at half the dose of gadolinium in the central nervous system as well as other body regions, Bracco said.