A variation on PET offers a new way to diagnose hypoxia in tumors. Researchers at the University of Tokyo and Japan's National Institute of Radiological Sciences have demonstrated that positronium, which forms in tissues due to positron emission from a radiopharmaceutical agent, decays differently depending upon its chemical environment, and it is especially sensitive to local oxygen saturation. This means that signs of tumor hypoxia could be spotted among the gamma rays collected routinely during PET imaging, providing clinicians with an additional source of information to guide treatment decisions.
Most positrons created during a PET scan are annihilated almost as soon as they are emitted: They lose energy through interactions with nearby molecules, then they collide with electrons in those molecules to produce pairs of 511 keV photons. Some positrons hang around a little longer, however, and instead of annihilating the electrons that they encounter, they capture them, forming metastable positronium atoms.
When this happens, the positronium is created in one of two distinct configurations. The least stable is parapositronium (p-Ps), in which the spins of the electron and positron point in opposite directions. p-Ps atoms have a mean lifetime of only 125 ps, after which they decay into a pair of 511 keV photons. This process therefore adds to the near-immediate gamma signal produced by annihilation of those positrons that never form a positronium atom.
The other configuration is orthopositronium (o-Ps), in which the spins of the electron and positron are parallel. Left alone, o-Ps would decay into three photons (with energies ranging from 0 to 511 keV) after a mean lifetime of 142 Newton seconds. This much longer period means that o-Ps atoms have more time to interact with their surroundings before they decay.
One of the routes open to o-Ps atoms is an interaction called spin exchange. In this process, the positronium's electron switches with an electron of opposite spin in a nearby molecule. This converts the positronium atom into the less stable p-Ps form, hastening its decay.
The likelihood of spin exchange occurring depends on the availability of unpaired electrons in the vicinity of the o-Ps atoms. In tissues, such unpaired electrons are present primarily in oxygen molecules. This means that in oxygen-poor environments such as hypoxic tumors, more o-Ps atoms survive long enough to decay via the three-photon route. Measuring the timing and spectrum of the gamma rays emitted during the PET procedure should, therefore, yield information about the oxygen saturation.
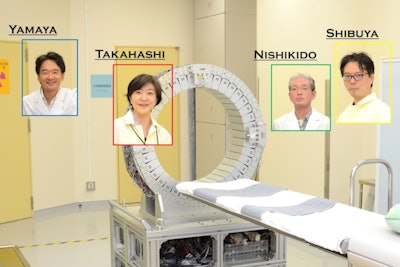 Researchers in Japan aim to use PET scans to detect oxygen concentration in tumors, which may lead to more effective cancer treatment. From left: Taiga Yamaya, PhD; Dr. Miwako Takahashi, PhD; Fumihiko Nishikido; and Kengo Shibuya, PhD. Image courtesy of Taiga Yamaya, CC BY 4.0)
Researchers in Japan aim to use PET scans to detect oxygen concentration in tumors, which may lead to more effective cancer treatment. From left: Taiga Yamaya, PhD; Dr. Miwako Takahashi, PhD; Fumihiko Nishikido; and Kengo Shibuya, PhD. Image courtesy of Taiga Yamaya, CC BY 4.0)As they reported in an article posted on 1 October 2020 in Communications Physics, Kengo Shibuya, PhD, and colleagues tested this principle by preparing samples of water saturated with either air, nitrogen or oxygen. Each sample also contained the unstable sodium isotope 22 (Na-22).
When Na-22 undergoes beta decay, it simultaneously emits a high-energy gamma ray at 1.27 MeV. The researchers used this signal as the starter pistol for each measurement. In instances where the positron emission resulted in a positronium atom, the end of the measurement was marked by the detection of sub-511-keV photons announcing the positronium's final decay.
By comparing the timing and energy of the photons emitted during millions of measurement intervals for the three samples, the team derived a linear relationship between oxygen saturation and positronium decay rate. They calculated that, in a clinical PET scanner, an acquisition time of around 30 minutes would yield enough measurements to distinguish a hypoxic tumor from normally oxygenated healthy tissue.
In terms of detector hardware, current PET devices are already suited to this task, although the researchers say that they will need new timer systems and software.
"The performance required by the new timers is comparable to those already used in conventional PET," says Shibuya. "Therefore, I think it will not be difficult for medical device manufacturers to install them."
Finding the right radiopharmaceutical agent might be more challenging, however. Whereas PET imaging techniques employ pure positron emitters, positronium imaging can only work if, like Na-22, the radioisotope emits a gamma photon and a positron simultaneously. Unfortunately, the 2.6-year half-life of Na-22 makes it unsuitable for the clinic, for which sources with half-lives on the order of hours or days are required.
Marric Stephens is a freelance science writer based in Bristol, U.K.