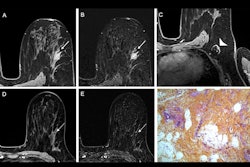
Bayer has submitted a marketing authorization application to the European Medicines Agency for its investigational contrast agent gadoquatrane.
The contrast agent is designed to boost contrast enhancement in MRI to find and visualize known or suspected pathologies in all body regions and the central nervous system in adults and pediatric patients, including neonates.
The submitted dose of 0.04 mmol gadolinium per kilogram body weight represents a gadolinium dose reduction of 60% compared with the standard of care macrocyclic contrast agents dosed at 0.1 mmol Gd/kg body weight, Bayer said.
Bayer recently announced the submissions of gadoquatrane in Japan and the U.S. Further regulatory applications to health authorities worldwide are planned for the coming months, the company said.
The company recently completed a phase III study showing that gadoquatrane is effective and safe for use in both adults and children.