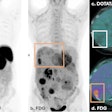
Orthopedic digital imaging developer EOS Imaging received clearance from the China State Food and Drug Administration (SFDA) to market its EOS product in the country.
The system is already in clinical use in several markets in the Asia-Pacific region, including Hong Kong, Singapore, Japan, Australia, and Vietnam. In addition, EOS equipment is installed in a Shanghai hospital, where it is being used for clinical studies, creating awareness for the technology in China.
The SFDA clearance will allow the company to develop commercial activity in China, which includes more than 1,200 top-grade hospitals, within an established distribution partnership, according to the vendor.