Insufficient attention is given to paraduodenal pancreatitis, a distinct form of chronic pancreatitis occurring predominantly in and around the duodenum along the minor papilla, according to award-winning research presented at this week's European Society of Gastrointestinal and Abdominal Radiology (ESGAR) annual meeting in Edinburgh, U.K.
A painful solid and/or cystic lesion along the pancreatic groove in a man aged between 40 and 50 with a history of chronic alcohol abuse and/or smoking should lead radiologists to consider paraduodenal pancreatitis, noted lead author Dr. Ankur Arora, assistant professor in the department of radiology/interventional radiology, Institute of Liver & Biliary Sciences (ILBS) in New Delhi, India. The classic clinical presentation is upper abdominal pain, vomiting, and weight loss.
"Awareness of the possibility that a paraduodenal solid/cystic lesion depicted on imaging studies may represent paraduodenal pancreatitis should influence the radiologist to avoid mislabeling the entity as a more ominous pancreatic or periampullary carcinoma," he stated. "Conversely, before making the diagnosis of paraduodenal pancreatitis, the possibility of malignancy should be carefully excluded."
Arora and his colleagues received the top scientific award, a magna cum laude, for their electronic poster at the ESGAR meeting, which began yesterday and runs until Friday. They described paraduodenal pancreatitis as "a lesser known radiological entity," for which the imaging modalities of choice are endoscopic ultrasonography and MRI with MR cholangiopancreatography (MRCP). Given the continuing rise in alcohol consumption globally, they anticipate that the disorder will become much more prevalent in the future.
Clinical and radiological diagnostic clues to differentiate groove carcinoma from solid variant of paraduodenal pancreatitis
|
|||||||||||||||||||||||||||||||||||||||||||||
| Source: Arora A, Mukund A, Bhatia V, et al. Paraduodenal pancreatitis: a radiologically less known entity. Poster presented as part of the ESGAR annual meeting, Edinburgh, U.K., 12-15 June 2012. | |||||||||||||||||||||||||||||||||||||||||||||
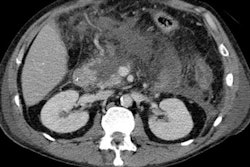
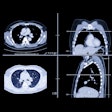
Paraduodenal pancreatitis is an umbrella term used for conditions that previously had a variety of names, including cystic dystrophy of pancreas, paraduodenal wall cyst, groove pancreatitis, pancreatic duodenal hamartoma, and myoadenomatosis. These patients have clinical characteristics similar to those of chronic pancreatitis, and characteristic imaging findings include a solid or cystic pattern corresponding to the path of the duct of Santorini/minor papilla (groove region) with thickening of the duodenal wall, Arora pointed out.
Paraduodenal pancreatitis can be classified morphologically as either a solid-mass occurring predominantly in and around the minor papilla (solid variant) or cystic lesions (cystic variant) within the pancreaticoduodenal groove or intramurally within the duodenal wall.
The solid variant is characterized by the presence of a sheet-like mass corresponding to the fibrous-scar predominantly located in the pancreaticoduodenal groove, the authors explained. The cystic variant is characterized by the presence of cysts in the duodenal wall (cystic dystrophy of the duodenum) and cysts within the pancreatico-duodenal groove (paraduodenal cysts) with or without inflammation, thickening, and fibrosis of the duodenum.
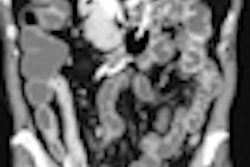
Abdominal ultrasound may be able to demonstrate a hypoechoic mass adjacent to the pancreatic head with thickening of the second part of the duodenum, while CT can reveal the histological characteristics of the disease, they noted. On CT, paraduodenal pancreatitis is seen as a hypoattenuating poorly enhancing soft-tissue mass in the pancreatico-duodenal groove (solid variant). The duodenal wall is frequently involved and thickened, and this may be accompanied with cysts in the duodenal wall and/or the groove (cystic variant).
"MRI can also readily demonstrate the pathologic features characteristic of the disease, i.e. the fibrous tissue or cysts in the pancreaticoduodenal groove, thickened and inflamed duodenal wall, and/or duodenal wall cyst formation. The most characteristic finding on MRI is sheet-like mass (solid subtype) corresponding to the fibrous scar in the groove. This exhibits hypointense signal relative to the pancreatic parenchyma on T1-weighted images with isointense/hypointense/or slightly hyperintense signal on T2-weighted images," stated the authors.
On contrast-enhanced imaging, the mass shows delayed enhancement reflecting its fibrous nature. Cystic lesions of the groove and/or the duodenal wall are well displayed on T2-weighted images.
Endoscopic retrograde cholangiopancreatography (ERCP) can demonstrate dilatation of the Santorini's duct and its branches, depicting intraductal stones or protein plugs. At present, ERCP is used mainly for endoscopic therapy, they said.
Endoscopic ultrasonography (EUS), on the other hand, can easily demonstrate the hypoechoic area between the duodenal wall and the pancreatic parenchyma,
thickening and narrowing of the duodenal lumen, associated pancreatic calcifications and pseudocysts, and stenosis of the common bile duct and/or dilatation of the pancreatic duct. Furthermore, the diagnosis can be confirmed by EUS-guided fine-needle aspiration in case the imaging findings overlap with groove pancreatic carcinoma, the authors added.
The differential diagnosis of the solid form of paraduodenal pancreatitis includes pancreatic carcinoma, cholangiocarcinoma, duodenal carcinoma, or acute pancreatitis with phlegmon along the groove. Cystic pancreatic lesion and pseudocyst along the groove are important diagnostic considerations for cystic paraduodenal pancreatitis. The most important differential diagnosis is pancreatic carcinoma arising in the groove region, but the distinction between fibrous tissue in solid paraduodenal (groove) pancreatitis and scirrhous carcinoma of the pancreatic head invading the groove is often difficult on CT and MRI, they noted.
"The distinction between the two, although difficult, is crucial as the appropriate management of the two conditions differs significantly. It is particularly challenging in those cases of pancreatic carcinoma which have a significant fibrous component and therefore may display delayed enhancement similar to that seen with groove pancreatitis," they explained.
Preoperative recognition is important to avoid unnecessary procedures, but pancreaticoduodenectomy still may be required to relieve obstructive symptoms, the authors have found. Surgical resection in the form of pancreatico-duodenectomy (using either the Whipple procedure or a pylorus preserving pancreaticoduodenectomy) is the preferred therapeutic option in symptomatic cases, and in confounding cases to rule out malignancy. Conservative medical management is suggested in the acute phase of paraduodenal pancreatitis, and endoscopic cyst drainage or stenting may also have been attempted in cases with biliary or duodenal obstruction, they concluded.