An original paper in European Radiology scans the many promising developments in stem cell regeneration of cardiac tissue following myocardial infarction.
"It has become clear that there is no single 'best method' in cell tracking," wrote Alessandro Ruggiero and colleagues from Erasmus MC: University Medical Center in Rotterdam, along with researchers from Memorial Sloan-Kettering Cancer Center in New York City (Eur Radiol, July 7, 2011).
Rather, there is an array of high sensitivity, high spatial resolution, and functional techniques that work best in combination. "The persistent trends in molecular imaging are: to focus on the development of novel MR-compatible probes able to monitor and track with sufficient sensitivity and specificity the fate of transplanted cells, new PET/SPECT reporter genes with lessened immunogenicity and oncogenicity issues, and the application of related radioprobes with better pharmacokinetic profiles," they wrote
The crux of the matter is that several distinct approaches -- each with its own strengths and weaknesses -- have clinical potential. Indeed, some methods such as multimodal imaging are very promising.
Nevertheless, progressing from experimental results into reliable clinical protocols is a tall order that will require much research into choosing, injecting, and -- for the radiology community, in particular -- visualizing autologous stem cells and their effect on heart tissue, according to the authors.
"Stem cell therapies hold the great promise and interest for cardiac regeneration among scientists, clinicians, and patients. However, advancement and distillation of a standard treatment regimen are not yet finalized," Ruggiero and colleagues wrote.
Significant research and clinical interest has been directed at stem cells for their potential to regenerate otherwise permanently damaged tissues. The research has given new hope for regenerative therapy following myocardial infarction.
Successful studies have shown stem cell-based therapy has the promise to limit the degradation of cardiac function after myocardial infarction. Beginning in 2002, studies have shown the successful intracoronary transplantation of autologous stem cells.
Mixed results are attributed to the lack of standardized protocols relating to "cell number, route and timing of injection, baseline patient characteristics, and techniques of evaluating cardiac function," the authors wrote.
In 2009, Dill et al used intracoronary-injected bone-marrow derived progenitor cells to improve left ventricular function after acute ST-segment elevation myocardial infarction in 204 patients, Ruggiero and colleagues noted. In patients injected with bone marrow cells, ejection fraction increased significantly by 3.2 ±1.3% at four months, and the improvement was sustained by +3.4 ± 1.3% at 12 months.
Moreover, recent meta-analyses have shown improvements in ejection fraction, ventricular dimension, and infarct area that while modest are statistically significant. "[T]echnical and protocol refinements have played a critical role not only in the evaluation of the recovery of cardiac function but also in providing important insights into the mechanism of action of stem cells," the authors wrote.
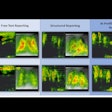
Molecular imaging "has rapidly become a necessary tool for the validation and optimization of stem cell engrafting strategies in preclinical studies," Ruggiero et al wrote. "These include a suite of radionuclide, magnetic resonance, and optical imaging strategies to evaluate noninvasively the fate of transplanted cells."
Imaging
Imaging is used to visualize and monitor the biodistribution of cells, and possibly monitor the differentiation status of implanted stem cells. Each method has its strengths and weaknesses.
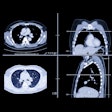
MRI is widely established for cardiac function analysis and generally uses paramagnetic contrast material to visualize the stem cells, the group wrote, owing to its excellent spatial (10-100 µm [small animal MRI]); 500-1500 µm [clinical]) and temporal resolution to visualize stem cells using superparamagnetic and paramagnetic agents.
Superparamagnetic iron oxide (SPIO) nanoparticles visualize the stem cells by acting as magnetic inhomogeneities that locally disturb the magnetic field, according to the authors. Cell uptake is controlled through the size and electrostatic charge conditions of the SPIO.
The advantages of this approach include biocompatibility and limited effect on cell function, as well as biodegradability. However, recent studies have shown evidence of marrow stem cell migration and colony-forming ability.
"A major issue beyond potential cellular effects is the question of contrast specificity to the presence of cells," Ruggiero and colleagues wrote. "Namely, the hypointense signal is maintained at a site regardless of cell viability and SPIO are present not necessarily within implanted stem cells at longer time points, but rather in phagocytosing monocytes following stem cell death."
SPIO-induced hypointensity can sometimes be difficult to interpret if obscured by the presence of endogenous blood derivates. Still, the use of iron oxides approaches "should not be discouraged" because of their high sensitivities, the authors wrote.
Paramagnetic ions or so-called "positive contrast" agents such as gadolinium chelates and manganese chloride are sometimes used to label cells, as they allow the visualization of stem cells as a hyperintense signal on T1-weighted images. Sensitivity is lower than with SPIO-labeled cells "as a result of reduced water accessibility to chelated ions following intracellular concentration, resulting in decreased relaxivity," noted the authors, and a number of methods aimed at driving the endosomal escape of paramagnetic compounds have been attempted to overcome this phenomenon. Safety issues related to the "rapid dechelation of compounds at the low pH of lysosomes and endosomes" are also a concern.
Submillimolar concentrations of manganese chloride (MnCl2) have enabled stem cell labeling and detection without detectable toxicity, the group added.
F-19 MR
F-19 can be used as the basis of the signal for MR spectroscopy and image formation. Although not yet widely implemented clinically, its advantages include the fact that F-19 is not present naturally in soft tissues, meaning its signal is derived exclusively from application of the exogenous contrast agent, the authors noted.
F-19 MRI can also be used with existing H-1 imaging hardware, and it can be overlaid on anatomic images for a high-resolution map of cell transplantation. F-19 cell tracking is in the early stage of development, but efforts are under way to improve sensitivity, and the technique is expected to play a future role, Ruggiero and colleagues wrote.
Radionuclide imaging
Radionuclide imaging offers the advantage of high sensitivity and high acute cell retention as a percentage of net injected dose. However, PET and SPECT suffer from inferior spatial resolution compared with MRI. In addition, radionuclide-labeled cells can only be visualized as long as the radioactivity is still detectable -- a significant limitation on the value of direct labeling for medium- and long-term stem cell transplant monitoring. SPECT, for example, has been used to monitor the short-term fate of transplanted cells labeled with compounds such as indium-111 oxine.
Large doses are needed to generate useful quantitative images within a reasonable time frame, raising the possibility of radiation damage in the form of reduced viability and proliferation of cells, as demonstrated by Brenner et al, the group noted.
PET has two to three orders of magnitude higher sensitivity versus PET, and F-18 FDG has been used for labeling and short-term imaging, though studies showed poor engraftment of stem cells.
PET/CT improves sensitivity beyond that of PET alone, enabling a combination of anatomical and functional imaging; however, "this multimodal imaging capability and clinical availability are tempered somewhat by the short half-life of F-18, driving a search for radioisotopes with a longer half-life such as copper-64" -- though only short-term results are possible with these agents as well.
Reporter genes
Reporter genes could potentially reveal insights into the mechanisms and fate of stem cell therapies, the researchers said. The method requires a combination of reporter transgene and reporter probe which interact, producing a signal that may be detected and quantified with the appropriate imaging technique.
Among its advantages, the reporter gene "identifies with exquisite specificity only viable cells" which actively contain the gene product, and allows long-term tracking of transduced stem cells, circumventing issues of probe dilution with cellular proliferation. Because the reporter genes' signal is always active, they can be used for the evaluation of transplantation, migration, and proliferation of stem cells, the authors wrote.
Wu et al pioneered reporter gene techniques by imaging in vivo transplanted cells (expressing luciferase or HSV1-sr39tk) in the heart for as long as two weeks using F-18 FHBG PET or bioluminescence imaging, they noted. Cao et al achieved survival and proliferation for up to four weeks in a mouse study.
Despite the advantage of signal amplification in HSV-tk-based approaches to reporter genes, immunogenicity may limit use of the technique in humans, though the use of other techniques may overcome this limitation.
Several important issues remain to be resolved. For example the nonphysiological expression of reporter gene proteins may disturb stem cells' cellular and therapeutic function, the authors explained.
Adenoviral transfection is hampered by episomal gene expression and by immunogenicity, defined as the "leakiness" of immunogenic adenoviral proteins that can lead to an immune response.
The use of lentiviral vectors has emerged as a potential solution that enables integration of the reporter gene in the host cell chromatin, allowing stable expression in dividing cells; however, transgene expression can be silenced by DNA methylation when strong promoters are used to drive expression of the reporter gene, the authors wrote.
"The integration of the reporter gene within the genome has raised concerns about the risk of mutagenesis and potential oncogenicity," they wrote. Potential MRI reporter gene candidates include β-galactosidase, transferrin receptor, ferritin, MagA, and lysine-rich proteins.
Optical imaging
Bioluminescence
Optical imaging techniques including bioluminescence, planar, and fluorescence-mediated tomography have been largely restricted to use in preclinical models, the group reported. The most common use for bioluminescence is cell tracking in stem cell transplantation studies.
Bioluminescence imaging has many advantages in that it is highly sensitive, quantitative, simple, and inexpensive, Ruggiero and colleagues wrote. However, the barrier to clinical use lies in its inherently poor tissue penetration of 1 to 2 cm, allowing only surface imaging, and its high scatter rates and low resolution (3-5 mm), which hamper the evaluation of the cells in their exact location, according to the authors.
Fluorescence
Labeling of stem cells with fluorescent probes has been driven by the availability of near-infrared (NIR) probes, whose spectral properties match the lower attenuation in the NIR window, allowing greater signal penetration, the team explained. As a result, they do have clinical potential that is limited to near-surface or intraoperative stem cell tracking.
NIR offers high sensitivity and tomographic capabilities, and intracoronary delivery of NIR dye IR-786 has been tracked in a swine model, the authors noted. Quantum dots (QDs) are fluorescent nanoparticles that have been used to label stem cells. To date, no adverse effects on the cells have been reported.
"One of the most attractive qualities of these nanoparticles is their capacity for multiplexed imaging," Ruggiero et al wrote. "The tracking of different cell populations is concurrently achieved by labeling cells with different QDs. Multiplex optical imaging of QD-labeled embryonic stem cells have been reported up to 14 days from injection in mice. Long-term effects on stem cell function are still unknown; however, there are concerns related to their metallic core.
Multimodal imaging
There is great interest in complementing the sensitivity of radionuclide or optical techniques with the high anatomical resolution of MRI, the group wrote.
"To date, the most interesting approach has been to develop transgenic cells that carry an optical/nuclear imaging reporter together with passive labeling with MRI contrast agents before administration," Ruggiero and colleagues wrote.
They cited a study by Qiao and colleagues, who assessed the survival and proliferation of SPIO-labeled murine ESC transduced with HSV1-sr39tk during four weeks following injection into the healthy or infarcted myocardium. In a human study, CD34+ mononuclear cells from umbilical cord blood were transduced with a reporter gene and labeled with SPIOs.
A recent optical, PET, CT, and MRI coregistration approach transduced CD34+ cells with a triple fusion reporter gene (e-GFP, f-Luc, and HSV1-tk), the authors wrote.
Bioluminescence imaging showed that cells persisted in the heart for up to 12 months and MRI showed improvement in the left ventricular ejection fraction for up to six months.
While there are many promising approaches, more work is required before a broad translation to clinical use can be achieved. Still unresolved are safety and ethical issues, questions about the best route of delivery, the optimal type and number of cells to be injected, and the optical timing, to name a few challenges, the authors wrote.