What are the pros and cons of contrast-enhanced mammography (CEM)? Which technique produces the best results? Addressing these questions has secured a prestigious prize for Spanish researchers.
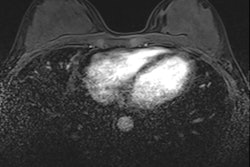
"CEM adds the value of functional behavior (contrast enhancement) to the morphological findings (mammography)," noted Dr. Rosa Lorente Ramos, PhD, a radiologist at the Unidad Central de Radiodiagnóstico CAM (UCR), Hospital Universitario Infanta Leonor, Madrid, and colleagues.
"CEM and MRI are based on tumor angiogenesis and contrast leakage from pathologic vessels into the tumor interstitium. Most malignant lesions show contrast enhancement," they pointed out.
The group gave these four top tips:
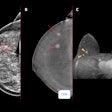
- When interpreting CEM, it is essential to interpret low-energy and recombined images together. The degree of suspicion of morphologic findings on the low-energy images determines the appropriate BI-RADS category.
- Lack of enhancement does not exclude malignancy. Enhancement may decrease in cases of ductal carcinoma in situ and certain invasive carcinoma (lobular and mucinous carcinoma).
- Benign causes of enhancement are atypical ductal hyperplasia, fibroadenoma, infection and inflammation, papilloma, pseudoangiomatous stromal hyperplasia, spindle cell lesions, complex sclerosing lesions, and radial scar.
- No enhancement at a site of low suspicion in low energy images suggests benign findings. If suspicious enhancement is identified on recombined images without a low-energy correlate, further workup is warranted. Calcifications with suspicious features on low-energy images should always undergo biopsy.
Protocols and technique
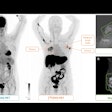
Some patients at the Madrid facility undergo CT and mammography (for evaluation of known malignancies, oncologic patient surveillance).
"Both studies are scheduled on the same day, and coordination among radiographers is mandatory to perform both exams with the same contrast (10 minutes availability of contrast for CEM)," Lorente Ramos and colleagues pointed out in an e-poster that received a cum laude award at RSNA 2021.
"When contrast material is injected intravenously in the CT scanner, the breast radiographer starts the chronometer in the mammography device. Immediately after the CT acquisition is finished, the patient is taken in a wheelchair to the mammography unit, where the CEM is performed," they continued.
The team prefers to use a power injector for contrast administration to obtain a rapid bolus of contrast and a bolus chaser of normal saline. An area for intravenous line insertion, designated space to monitor patients for adverse reactions, and a standard contrast reaction kit and crash cart are essential. Patient evaluation should focus on history of allergic reactions, renal function, weight, and pregnancy.
"With the breast out of compression, nonionic low-osmolar iodinated material (350 mg l/mL or 300 mg l/mL) is injected intravenously at a standard dose of 1.5 mL/kg and a rate of 3 mL/s," they explained.
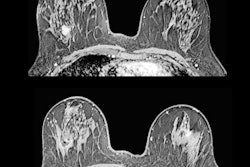
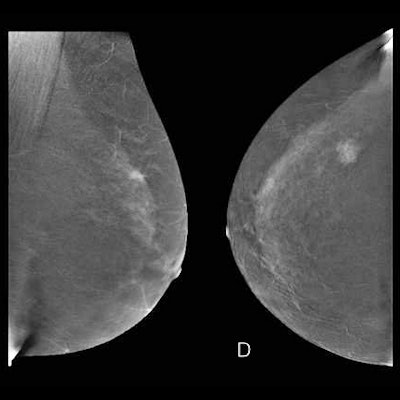
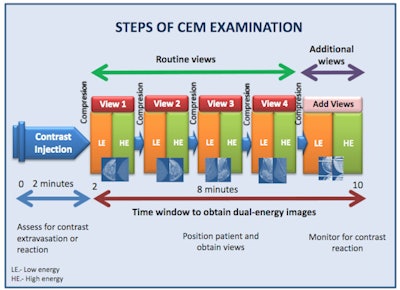 The graphic illustrates the various stages of the CEM examination.
The graphic illustrates the various stages of the CEM examination.Two minutes after the start of the injection, the breast is placed into compression and paired low-energy (23-33 kVp) and high-energy (44-50 kVp) images are obtained in standard craniocaudal and mediolateral oblique views. Additional projections may be obtained during the first 10 minutes from injection. Recombined images are obtained by the substraction of the low-energy images from the high-energy images.
Image evaluation is in two parts:
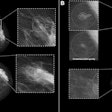
- Morphological assessment. The low-energy images are similar to a standard digital mammogram, allowing morphological evaluation of lesions that should be performed first.
- Functional assessment. Recombined images should be analyzed. Signal from background breast tissue is cancelled, highlighting areas of iodine uptake.
For reporting, ACR BIRADS is preferred. The group uses standardized terminology for mammography for low-energy images, and then MRI terminology for recombined images.
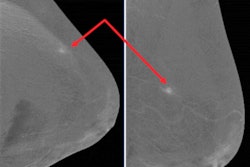
"Be aware of lesions visible in only one view. They may be cancer," the authors warned. "Lesions need to be included in the field-of-view to be imaged. Blind areas may appear (axillary tail, areas of breast high on the chest wall or inferior medial). Some cases require additional projections."
Pitfalls may be related to technical difficulties. For instance, a mass may be obscured by breast implants. Using the Eklund technique may help by displacing the implant and making the lesion apparent both on digital mammography and CEM, they advised.