The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) have released a new practice guideline for somatostatin receptor (SSTR) PET imaging of patients with neuroendocrine tumors (NETs).
The guideline will help physicians recommend, perform, interpret, and report results of SSTR-PET imaging studies for patients with NETs. It was published in the February issue of the Journal of Nuclear Medicine.
"Adequate precision, accuracy, repeatability, and reproducibility are essential for the clinical management of patients and the use of SSTR PET within multicenter trials," wrote first author Dr. Thomas Hope of the University of California, San Francisco. "A standardized imaging procedure will help to promote the appropriate use of SSTR PET and enhance subsequent research."
SSTRs are overexpressed on a wide range of NET cells. PET imaging of abnormal SSTR activity has largely replaced scintigraphic imaging using indium-111 pentetreotide (OctreoScan), and this change has been driven by the development of a new generation of radiotracers, the authors wrote.
Specifically, SSTR PET imaging with gallium-68 (Ga-68) DOTATATE, Ga-68 DOTATOC, and copper-64 (Cu-64) DOTATATE can improve the sensitivity of lesion detection, lower radiation dose, and provide shorter and more convenient study durations. The new guideline focuses on these PET radiotracers.
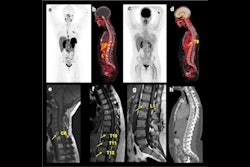
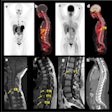
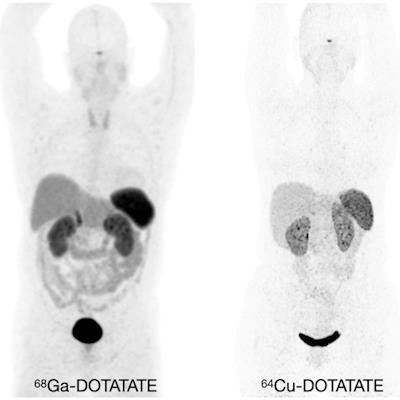
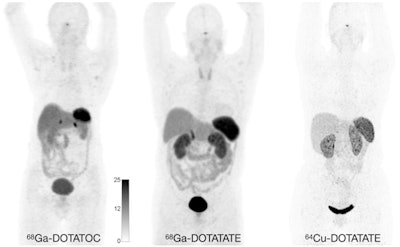 Normal biodistribution in patients of the radiotracers Ga-68 DOTATOC, Ga-68 DOTATATE, and Cu-64 DOTATATE. Image courtesy of the Journal of Nuclear Medicine.
Normal biodistribution in patients of the radiotracers Ga-68 DOTATOC, Ga-68 DOTATATE, and Cu-64 DOTATATE. Image courtesy of the Journal of Nuclear Medicine.The guideline addresses common clinical indications, qualifications and responsibilities of personnel, specifications of the examination, documentation and reporting, and dosimetry. It also presents standardized quality control procedures for SSTR PET, as adequate precision, accuracy, repeatability, and reproducibility are essential for the clinical management of patients and the use of SSTR PET within multicenter trials, the authors noted.
The SNMMI and EANM periodically define new standards and guidelines for nuclear medicine practice to help advance the science of nuclear medicine and to deliver effective and safe medical care to patients. Standards and guidelines representing policy statements by the organizations go through a thorough consensus process in which they are subjected to extensive review.
In addition to Hope, co-authors included 10 experts from eight U.S. institutions and European experts in Germany, France, and Denmark.