An introduction to medical device software: regulations and requirements to include EU and FDA guidance and risk management.
Medical Device Software: Complying With the MDR and FDA Regulations
Nov 19th, 2019Nov 20th, 2019
London, 17
GB
Phone:2077494730
Latest in Home
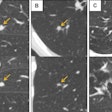
DL algorithm estimates lung nodule cancer risk, reduces false positives
September 18, 2025
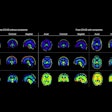
Neuroinflammation persists for 2 years in long COVID
September 17, 2025
ChatGPT gathers momentum in MR imaging
September 16, 2025
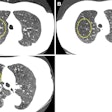
Low-dose CT ties emphysema to mortality in previous smokers
September 16, 2025