This one-day practical workshop will introduce you to the concept of postmarket surveillance, what it is, how to implement it effectively, and how to understand and interpret the regulatory requirements for your products.
Introduction to PMS and the European Medical Device Vigilance System
Sep 16th, 2014
Latest in Home
MRI safety panel unveils findings on patient death
February 13, 2026
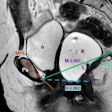
Abdominal pain? Consider fishbones
February 13, 2026
Christiane Kuhl endorses DRG's new women’s health campaign
February 13, 2026
Do young athletes with low back pain need advanced imaging?
February 12, 2026