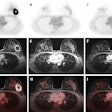
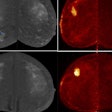
The world’s top nuclear medicine societies have issued international guidelines on the role of PET/CT in patients with invasive ductal carcinoma breast cancer, with the document released on 14 May in the European Journal of Nuclear Medicine and Molecular Imaging.
Experts from the European Association of Nuclear Medicine (EANM) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI) said that despite much literature about the role of F-18 FDG-PET/CT in these patients, “there exists no international guideline with involvement of the nuclear medicine societies about this subject.”
Thus, the group reviewed the current literature, held discussions, and jointly summarized the role of F-18 FDG-PET/CT in women with invasive ductal carcinoma (now called breast cancer “of no special type”) according to the evidence.
They found that F-18 FDG-PET/CT can be useful in breast cancer management, including initial staging, assessing neoadjuvant systemic treatment response, assessing treatment response in the metastatic setting, searching for loco-regional or metastatic recurrence, and re-staging after therapy, as well as radiation therapy planning.
The authors’ key recommendations include the following:
- Quantitative PET features (SUV, MTV, TLG) are valuable prognostic parameters.
- In baseline staging, F-18 FDG-PET/CT plays a role from stage IIB through stage IV.
- When assessing response to therapy, F-18 FDG-PET/CT should be performed on certified scanners and reported either according to PERCIST, EORTC PET, or EANM immunotherapy response criteria.
- F-18 FDG-PET/CT may be useful to assess early metabolic response, particularly in nonmetastatic triple-negative and HER2+ tumors.
- F-18 FDG-PET/CT is useful to detect the site and extent of recurrence when conventional imaging methods are equivocal and when there is clinical and/or laboratorial suspicion of relapse.
“This guideline provides practical recommendations to be applied in clinical practice,” the group wrote.
The guideline should be viewed as a dynamic document in a constantly evolving field, rather than as a definitive document or summary of existing protocols, the authors noted. Moreover, modifications may be considered according to local legislation and regulations, they added.
The document has been endorsed by the American College of Radiology, the European Society of Surgical Oncology, the European Society for Radiotherapy and Oncology, the European Society of Breast Imaging/European Society of Radiology, and the European Society of Breast Cancer Specialists.
“F-18 FDG-PET/CT is extremely useful in [breast cancer] management, as supported by extensive evidence of its utility compared to other imaging modalities in several clinical scenarios,” the group concluded.
Corresponding authors included nuclear medicine physician Dr. Sofia Vaz, of the Champalimaud Clinical Center in Lisbon, Portugal, and breast imaging expert Dr. Elizabeth Dibble, of Brown University in Providence, Rhode Island, U.S.
The full article is available here.