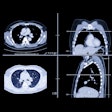
A single bottle of contrast agent contaminated with Klebsiella bacteria was the cause of eight patients becoming unwell after undergoing CT on 27 August at AZ Sint-Blasius Dendermonde in East Flanders, according to a media report.
After the incident, three patients were admitted to the intensive care unit, but they have since been transferred to a regular residential ward, stated an article posted on 1 September by Het Nieuwsblad, a Flemish newspaper. Two more patients also remain in the hospital, while three others were able to return home before the weekend.
“Thanks to the efforts of our internal physicians and staff, who went to great lengths to determine the cause, we were able to find the source of the infectious symptoms, specifically a contamination of the contrast agent with Klebsiella bacteria,” Peter Van Puyvelde, acting general manager of Sint-Blasius, told Het Nieuwsblad. “We take this very seriously and deeply regret that this could happen.”
AuntMinnieEurope revealed in a 28 August article that contamination was the likely cause, but how the contamination occurred cannot yet be determined, the Het Nieuwsblad article stated. It might have occurred at the facility of the contrast agent supplier, during storage, or during administration in the hospital, and none of these three possibilities has been ruled out or proven so far, it noted. The CT injector is a potential source.
 Microscopic photography of Klebsiella pneumoniae bacterial cells isolated on plain white background.Adobe Stock and Zachary.
Microscopic photography of Klebsiella pneumoniae bacterial cells isolated on plain white background.Adobe Stock and Zachary.
Klebsiella is a bacterium found in the intestines, the article explained. It is part of the normal flora in the human body and generally does not cause disease, according to the National Institute for Public Health and the Environment (RIVM). Patients with compromised health due to chronic lung diseases, injuries, those recovering from surgery, or those hospitalized, can become infected with Klebsiella, and the bacteria can be transmitted through direct contact via the hands, Het Nieuwsblad reported.
Fallout from the incident
An expert source told AuntMinnieEurope on 2 September, “The incident underlines the importance of strict control of the storage, use, and administration of contrast media. It suggests we need a revision of infection prevention protocols in radiology (e.g., single-use materials, temperature control of injection systems) and close supervision of internal supply and actions by staff.”
Contrast agents are susceptible to contamination, especially if injection systems are stored or used incorrectly, but this finding is very rare, the source added. “Similar outbreaks of Klebsiella in hospitals can often be linked to contaminated medical instruments (such as endoscopes), environmental reservoirs (e.g., washbasins), or infection clusters in intensive care units -- but not specifically to contrast agent injections.”
It seems likely that the contamination occurred during the handling of the vial in the radiological procedure, Dr. Francisco Vega, PhD, from the Department of Allergy, Hospital Universitario de la Princesa, Madrid, told AuntMinnieEurope. Because only one vial was contaminated, but eight patients were affected, it appears that a multidose vial was used, he noted. "The source of contamination could have been a breach in the sterility of the infusion system, whereby introducing one of its ends through the stopper of the radiological contrast vial may have led to contamination."
The isolation of Klebsiella also supports the hypothesis of faulty handling, given that this bacterium is highly prevalent in hospital settings and is responsible for many healthcare-associated infections, he added.
Another source, who is an expert in contrast media safety, thought that the risk of contamination may be related to the tube material and/or the handling, given that the tube must be changed daily, with every patient needing another line.
The source pointed out that two patients died of meningitis caused by Pseudomonas aeruginosa in a hospital in Germany in July 2001, their infections having been due to a contaminated contrast media (iomeprol [Imeron]) used as a multiple-dose vial over eight days. The findings appeared in an article published by the American Journal of Infection Control in February 2004.
Also of relevance here are these two articles: “Microbiologic contamination and time efficiency of use of automatic MDCT injectors with prefilled syringes,” published in February 2010 in the American Journal of Roentgenology, and "Bacterial contamination of contrast media stored after opening," published in July 1990 by the British Journal of Radiology.
Iomeron, from Bracco, was the contrast agent used on the eight patients. AuntMinnieEurope asked the company for its response to the article in Het Nieuwsblad and received this statement:
"Recently Bracco has been informed of a cluster of 8 consecutive cases of adverse events which occurred at the AZ Sint-Blasius Hospital in Dendermonde, Belgium. To determine its root cause and scope, Bracco immediately launched an investigation.
As to the disposables and bottles of Iomeron used in the 8 cases, we necessarily had to rely on the information obtained from the hospital that up to now kept the materials in its possession in view of its own investigation. In parallel, we have initiated an in-depth product investigation at our manufacturing plant and throughout our logistic network.
At this moment and in the light of the available information, the Klebsiella contamination appears to be isolated and limited to: i) a single hospital, ii) a single day, iii) a limited temporal window (2 hours in the morning of that day) and (iv) eight consecutive patients treated during that window.
No cases of Klebsiella contamination were observed before or after that limited (2 hour) time frame at the AZ Sint-Blasius Hospital nor at other hospitals that had used samples of Iomeron 400. In any event, no quality issue possibly deriving from the manufacturing of the product has been identified at Bracco.
By means of standard precautionary measure, the Belgian regulatory authority nevertheless requested that Bracco quarantines samples of a specific batch of Iomeron 400.
Meanwhile, the AZ Sint-Blasius Hospital publicly declared that the root cause of the observed cluster of adverse events was the bacterial contamination of the contrast material. However, the precise source of the contamination remains unknown at this time; the hospital itself also publicly stated that it could have originated from the administration process or the stocking procedure as well. Of note, the involved batches of Iomeron 400 did not show any quality issue and were sterile when released from the manufacturing site.
Accordingly, the source of the contamination is still being further investigated and Bracco is determined to identify the exact cause of the adverse events, as patient and product safety is our highest priority."