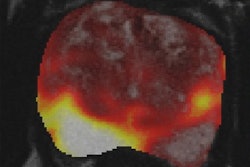
Molecular imaging diagnostics firm Blue Earth Diagnostics has received marketing authorization from the European Commission for its Axumin (fluciclovine F-18) PET agent.
The authorization covers the use of Axumin in PET imaging to detect prostate cancer recurrence in adult men with a suspected recurrence based on elevated blood prostate specific antigen (PSA) levels after primary curative treatment, according to the company. Blue Earth said it's now in discussions with potential manufacturing and distribution associates to make Axumin commercially available across Europe.
Axumin will initially be available in Norway, with additional countries planned in 2018, the firm said.