Radiopharmaceutical developer Ion Beam Applications (IBA) and drug company Wilex recently met with the U.S. Food and Drug Administration (FDA) to discuss the regulatory review process for IBA's Redectane PET/CT radiopharmaceutical.
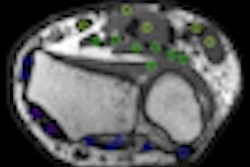
IBA and Wilex recently completed the phase III REDECT trial to determine whether the combination of the radiolabeled antibody Redectane with PET and CT could improve the diagnosis of renal masses, compared with the use of CT alone.
The trial achieved its primary end point, indicating that PET/CT with Redectane correctly diagnosed the presence of clear cell renal cell cancer with a sensitivity of 86% and sensitivity of 87%, findings that reached statistical significance, according to IBA.
The FDA confirmed that the trial provides reasonable evidence for the diagnostic efficacy and safety of Redectane, but suggested that IBA and Wilex consider an additional outcomes-based study to provide evidence of clinical benefit before submitting a biologics license application (BLA). IBA and Wilex, however, would prefer that such a study be conducted as a phase IV trial after Redectane reaches market.
IBA said that it would discuss the issue with its medical advisory board and then the FDA, and then make an announcement regarding its regulatory plan.
The companies and the agency also discussed concerns regarding the manufacturing of Redectane. Wilex has completed two consecutive lots for process validation of the antibody girentuximab at Avid Bioservices. The third production run was started before the meeting and is expected to be completed soon.
In addition, the FDA has requested from IBA data pertaining to the commercial production of Redectane: in particular, product characterization and process validation.