This seminar will provide essential guidance on how to undertake a successful conformity assessment process and subsequently apply (or continue to apply) the CE mark.
Medical Device Conformity Assessment Under the European Medical Devices Regulation (EU) 2017/745: Obtaining the CE Mark
Mar 25th, 2019Mar 26th, 2019
London, --
GB
Phone:+442077494730
Latest in Home
Do young athletes with low back pain need advanced imaging?
February 12, 2026
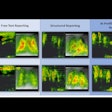
Structured reporting, AI improve chest x-ray workflows
February 11, 2026
Radiologist outrages public decency
February 10, 2026
'Hub-and-spoke' model and telemedicine can boost stroke care
February 9, 2026