A highly respected molecular imaging research group from Austria has reported that neoadjuvant chemoimmunotherapy leads to a high rate of pathologic complete remissions in cases of early-stage, non-small cell lung cancer (NSCLC). However, residual tumor size and changes in tumor size do not necessarily correlate with histopathological response, they say.
"Metabolic examinations such as [F-18] FDG-PET/CT better assess treatment response compared to morphological changes," noted Dr. Daria Kifjak; Dr. Lucian Beer, PhD; and colleagues from the Department of Biomedical Imaging and Image-Guided Therapy at Medical University of Vienna. They emphasized, however, that in addition to certain side effects, neoadjuvant chemoimmunotherapy can in rare cases induce unusual tumor reaction patterns such as a nodal immune flare, which involves immune-mediated increased lymphatic FDG uptake and/or increased lymph node size.
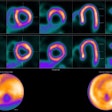
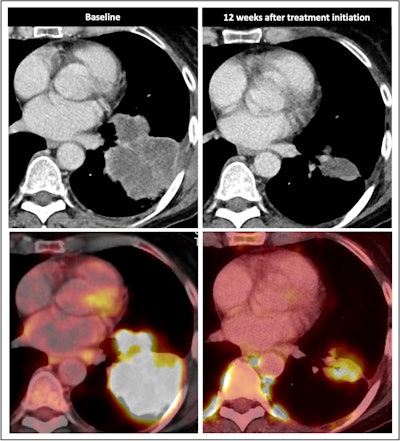
![Nodal immune flare: 72-year-old patient with NSCLC received a neoadjuvant treatment with three cycles of ICIT + C (carboplatin/pemetrexed/pembrolizumab). 12 weeks after treatment began, the patient showed an increase in size and FDG avidity but also major pathologic response with less than 10% of vital tumor burden (ypT1a, No, Ro [local]). All figures courtesy of Dr. Daria Kifjak and Dr. Lucian Beer.](https://img.auntminnieeurope.com/files/base/smg/all/image/2024/02/2024_02_22_Molecular_Insider_slide3.65d786b404f26.png?auto=format%2Ccompress&fit=max&q=70&w=400) Nodal immune flare: 72-year-old patient with NSCLC received a neoadjuvant treatment with three cycles of ICIT + C (carboplatin/pemetrexed/pembrolizumab). 12 weeks after treatment began, the patient showed an increase in size and FDG avidity but also major pathologic response with less than 10% of vital tumor burden (ypT1a, No, Ro [local]). All figures courtesy of Dr. Daria Kifjak and Dr. Lucian Beer.
Nodal immune flare: 72-year-old patient with NSCLC received a neoadjuvant treatment with three cycles of ICIT + C (carboplatin/pemetrexed/pembrolizumab). 12 weeks after treatment began, the patient showed an increase in size and FDG avidity but also major pathologic response with less than 10% of vital tumor burden (ypT1a, No, Ro [local]). All figures courtesy of Dr. Daria Kifjak and Dr. Lucian Beer.
The researchers analyzed radiological response patterns in patients with early-stage NSCLC receiving neoadjuvant chemoimmune checkpoint inhibitor therapy (ICIT). They found radiological response may be consistent or inconsistent with pathological response.
"Some patients with partial radiological response show a poor pathological response compared to others who show a complete pathologic response," they stated. "Residual tumor size and changes (e.g., decrease) in tumor size do not necessarily correlate with histopathologic response."
The authors explained that ICIT plus chemotherapy increases event-free survival compared with chemotherapy alone: 31.6 months versus 20.8 months; p = 0.0005. Among the patterns of pathological response are complete pathological response (cPR) involving no vital tumor cells, major pathological response (MPR) involving less than 10% vital tumor cells, and pathologic nonresponse involving more than 10% residual viable tumor cells.
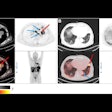
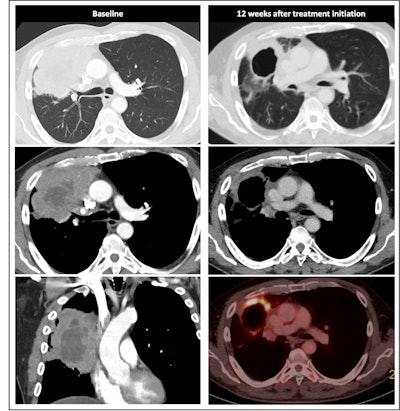
 Radiological response inconsistent with pathological response: 69-year-old patient with non-small cell lung cancer (NSCLC) received a neoadjuvant treatment with three cycles of immune-checkpoint inhibitor therapy plus chemotherapy (ICIT + C, carboplatin/pemetrexed/pembrolizumab). 12 weeks after treatment began, the patient showed complete pathologic response (ypTo, pNo) but only partial radiological response.
Radiological response inconsistent with pathological response: 69-year-old patient with non-small cell lung cancer (NSCLC) received a neoadjuvant treatment with three cycles of immune-checkpoint inhibitor therapy plus chemotherapy (ICIT + C, carboplatin/pemetrexed/pembrolizumab). 12 weeks after treatment began, the patient showed complete pathologic response (ypTo, pNo) but only partial radiological response.
The researchers noted in a poster presentation at RSNA in Chicago that a minority of patients with cPR show complete radiological response (24% vs. 1.1%). Also, ICIT leads to inflammatory cell infiltration with edema and later on tumor cell necrosis or fibrosis, the authors continued. Initially, this may lead to no change in lesion size, increase or appearance of new lesions (pseudoprogression), and mimicking of residual disease.
Most patients will have a residual tumor and fibrotic tissue, leading to insufficient assessment with standard-of-care imaging, while major pathologic response involves a reduction in tumor size with residual tissue and no major pathologic response means a reduction in tumor size or stable disease.
In NSCLC patients, baseline imaging can help determine clinical tumor stage, thereby ruling out distant metastasis and comorbidities. The main choices are F-18 FDG-PET/CT or contrast-enhanced MRI of the brain. Presurgical follow-up imaging can assist with assessment of operability (for the surgeon), histopathological workup and correlation (for the pathologist), and evaluation of adverse events of ICIT (for the pulmonologist).
 Radiological response inconsistent with pathological response: 75-year-old patient received a neoadjuvant treatment with three cycles of ICIT + C (carboplatin/pemetrexed/pembrolizumab). 12 weeks after treatment began, the patient showed complete pathologic response (ypTo, pNo) but only partial radiological response.
Radiological response inconsistent with pathological response: 75-year-old patient received a neoadjuvant treatment with three cycles of ICIT + C (carboplatin/pemetrexed/pembrolizumab). 12 weeks after treatment began, the patient showed complete pathologic response (ypTo, pNo) but only partial radiological response.
Therapy-related adverse events tend to be low incidence, mostly grade 1 and 2 events, and may be attributed to increased T-cell activity, auto-antibodies, and inflammatory cytokines, they pointed out. These events may have an impact on multiple organs and organ systems (endocrine, gastrointestinal, nervous, and musculoskeletal systems as well as the skin, lung, and liver). The most common conditions are rash (8.5% of patients), diarrhea/colitis (2.3%), and pneumonitis (1.1%).
An important limitation of F-18 FDG-PET/CT is its restricted ability to identify patients with cPR, according to the researchers. Also, patients with partial metabolic response can have either cPR, MPR, or non-MPR, and the technique fails to distinguish between true nodal progression and the so-called nodal immune flare/sarcoid-like reaction. Additionally, RECIST 1.1 and iRESCIST 1.1 are of limited value as they are neither designed nor validated to be applied in the setting of neo-adjuvant ICIT+C, they concluded.
The co-authors of the study were Drs. B.H. Heidinger, R.-I. Milos, M. Hochmair, Oguz Lafci, and H. Prosch.