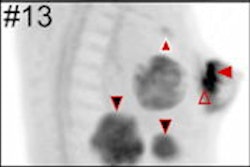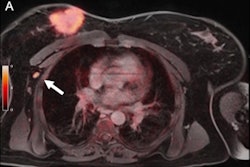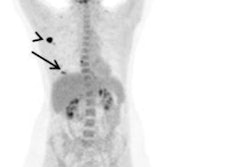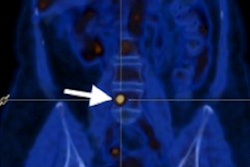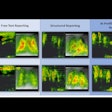
The clinical utility of F-18 fluoroestradiol PET imaging is continuing to evolve rapidly in problematic cases of breast cancer, award-winning researchers have demonstrated.
F-18 fluoroestradiol (FES) is a novel PET tracer designed to detect estrogen receptors, which are expressed in over 75% of breast cancers and have a central role in echogenesis, in addition to prognostic and therapeutic implications, explained Dr. Jeremy Ong, radiologist and nuclear medicine physician, and colleagues at the Peter MacCallum Cancer Centre in Melbourne, Australia.
 Peter MacCallum Cancer Centre, commonly abbreviated as Peter Mac, is an oncology research institute, cancer treatment, and professional oncologist training facility established in 1949. Picture courtesy of Alexander Cimbal / Alamy Stock Photo.
Peter MacCallum Cancer Centre, commonly abbreviated as Peter Mac, is an oncology research institute, cancer treatment, and professional oncologist training facility established in 1949. Picture courtesy of Alexander Cimbal / Alamy Stock Photo."FES PET/CT facilitates in vivo, whole-body evaluation of estrogen receptor blockade/degradation while on treatment. Personalized dose adjustments can be made," they stated in an e-poster presentation that received a cum laude prize at RSNA 2021. "Pharmacodynamic evaluation for optimal dosing with FES PET/CT has been implemented in Phase I clinical trials."
The PET/CT images are acquired around one hour after intravenous tracer injection, and most of the tracer is cleared by the liver and excreted with bile, similar to endogenous estradiol.
"No serious adverse events have been documented with FES PET," noted Ong, who currently works as a molecular imaging and radionuclide therapy fellow.
FES PET/CT is approved by the U.S. Food and Drug Administration (FDA) as an adjunct to biopsy to guide which lesions to biopsy or as a fallback if biopsy is not feasible. The technique evaluates in situ estrogen receptor binding and can guide optimal biopsy targeting. Lymph nodes can exhibit detectable estrogen receptor expression at very small sizes, below evaluable limits on CT.
The clinical uses of FES PET in breast cancer include the following:
- Detection of estrogen receptor sites and as a guide to biopsy
- Pharmacodynamic/dose adequacy evaluation of estrogen receptor directed therapy
- Complementing FDG-PET in the detection and characterization of disease heterogeneity, as well as identifying treatment resistance. "Complementary FDG/FES PET imaging demonstrates the large burden of multi-organ estrogen receptor disease that will not be controlled by targeted therapy alone and will dictate prognosis," the authors wrote.
- Problem-solving new metastatic lesions in breast cancer patients with co-existing malignancies.
The widespread use of FDG-PET imaging in oncology means FES-PET often has a complementary role in resolving clinical dilemmas.
"Clinical dilemmas where FES PET has documented clinical impact include determining the etiology of metastasis with coexisting malignancies, equivocal conventional imaging findings, and identifying estrogen receptor-plus disease and as an adjunct to biopsy," commented Ong et al. "In these situations, FES PET/CT findings inform management decisions in up to 93% of cases."
The same authors also won a cum laude award for their RSNA 2021 e-poster, "Tumor Heterogeneity: Implications for Theranostics and Beyond."
Editor's note: the PET image used to introduce this article on the homepage is of an 84-year-old woman with a multifocal cancer in the left breast and multiple bone FDG-avid metastatic lesions. Image courtesy of Surgical Oncology and Dr. Tima Davidson, Tel Aviv University, Israel.