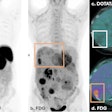
Advanced Accelerator Applications (AAA) has received orphan drug designation status for gallium-68 DOTATATE.
Both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) granted the designation for use of the radiopharmaceutical as a diagnostic agent for managing gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
AAA said the designation should help accelerate development of the agent for the benefit of GEP-NET patients in the U.S. and Europe.
Gallium-68 DOTATATE will be prepared using AAA's patented kit, which is reconstituted in hospital radiopharmacies without the use of a radiochemistry module. The product would be available to all hospitals, even facilities that do not have a fully equipped GMP production radiopharmacy unit.
In other company news, AAA has entered into an agreement to acquire Imaging Equipment (IEL), a privately held U.K. distributor of nuclear medicine products and technologies.
The purchase will be made using newly issued AAA shares, and as part of the transaction IEL's founding shareholders and top management will become shareholders in AAA, which is based in Bourg-en-Bresse, France.
The acquisition gives AAA its first direct presence in the U.K. and Ireland, the company said. It will also gain the rights in the U.K. and Ireland to IEL's licensed SPECT radiopharmaceutical diagnostic product, IELMag3, which is used to image the kidneys and urinary tract. IEL is headquartered in Chilcompton, Somerset.