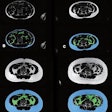
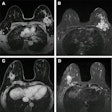
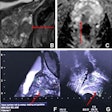
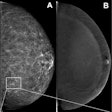
Dr. Olga Kubassova is an example of a new breed of global medical imaging entrepreneur who is thriving as a result of the host of opportunities in healthcare IT. She founded the U.K.-based Image Analysis, which specializes in developing tools for computer-aided analysis of complex medical datasets such as dynamic contrast-enhanced (DCE)-MRI and MRI acquired from patients with musculoskeletal conditions such as rheumatoid arthritis, and other inflammatory conditions.
Creating a company was a gradual and logical process, she noted. After completing two masters degrees simultaneously in math and IT at St. Petersburg State University in Russia and Lappenranta Technology University in Finland, Kubassova decided to pursue further studies in the field of healthcare.
 Data gained from advanced visualization will in the near future help general practitioners improve patient management, according to Dr. Olga Kubassova.
Data gained from advanced visualization will in the near future help general practitioners improve patient management, according to Dr. Olga Kubassova."I could have gone into law or finance after my master's degrees, but it was meeting healthcare staff who had a sense of doing something 'worthwhile' that inspired me to do something 'worthwhile' as a mathematician: specifically helping clinicians often overloaded with information to work faster without impairing accuracy," Kubassova said.
Her doctorate from the University of Leeds in the U.K. between 2004 and 2007 in "Analysis of dynamic contrast-enhanced MRI data sets" involved creating algorithms to eliminate patient motion from MRI exams to increase data quality. After this, the work focused on computer-aided detection (CAD) algorithms to enable clinicians to process dynamic MRI data more quickly than before to obtain quantitative machine-generated -- and therefore repeatable and objective -- answers instead of subjective reader-dependent opinions.
"From the very beginning I was working with specialist radiologists to solve real problems for real people," she explained. "Doctors were really using the tools and when the PhD ended, nobody wanted me to drop the subject, so in late 2007 we continued on a commercial basis."
With just Kubassova as its sole staff, in 2008 the company sold the licenses for the first prototype software, Dynamika-RA, to Abbott Laboratories as the basis for a project in early inflammatory disease detection and assessment of treatment effect.
The project is still ongoing and extension to software functionality allows analysis of any dynamic MRI data independent from scanner manufacturer, PACS provider, or imaging sequence. Along the way Kubassova picked up prizes as Young Entrepreneur of the Year in 2009 and Female Entrepreneur of the Year in 2011.
Dynamika, which was awarded "Innovation of 2011" in regional and national U.K. competitions, is now being used in a wide range of musculoskeletal inflammatory conditions, multicenter trials, and clinical research and practice. While initially designed for musculoskeletal MRI, Dynamika can also be applied to gastroenterology, neurology, and oncology.
Quantification of radiological data using CAD or advanced visualization software allows for a fast and reliable way of processing medical images. An automated and objective second opinion can challenge or affirm human diagnosis to speed up clinical or research workflow, and also allows clinical trials to be conducted in a more efficient manner through timely evaluation of treatment. The challenge for Kubassova and her colleagues is to determine how this technology can affect patient management.
Expansion in the field of advanced visualization and CAD is inevitable, and in the future general practitioners -- not just key consultants and radiologists -- also will benefit from prescribing and evaluating treatments based on quantitative data, she said.
Now with seven full-time employees on the R&D team and seven full- and part-time sales and marketing staff, the company will dedicate the rest of this year to consolidating its European market with 2012 earmarked for pursuing U.S. Food and Drug Administration approval and going global. She sees the interest of multinationals in smaller niche players as a natural progression.
"It's easier for smaller companies to develop something specific and tailored for practical application. The next step for them is to either go bankrupt, grow, and/or be acquired," Kubassova said. "Of course, if one day the opportunity presents itself, we may decide on the latter route, but for now we are really enjoying working with an interesting customer base."