Multiparametric MRI and radiomics can help predict distinct human epidermal growth factor receptor 2 (HER2) expressions of breast cancer, a research team from the Curie Institute in Paris has reported.
A team led by Toulsie Ramtohul, MD, noted that the radiomic signature and tumor descriptors from multiparametric breast MRI showed high predictive value for predicting HER2 expression levels, a capability that could have therapeutic implications.
"The results of this dual-center study showed that a multiparametric MRI-based radiomic signature ... could represent a noninvasive method for identifying patients eligible for HER2-targeted therapies," Ramtohul and colleagues wrote in an article published on 1 August in Radiology.
Conventional pathologic features are relied upon to help discriminate between HER2-positive, luminal, and triple-negative breast cancers. About half of breast cancers show low expression levels of HER2 and can be targeted by new antibody-drug conjugates.
Previous research suggests that multiparametric breast MRI radiomics of breast lesions and surrounding margins have ties with molecular subtype and levels of Ki-67, a prognostic biomarker for invasive breast cancer.
Ramtohul and co-authors wanted to find out whether multiparametric MRI-based radiomic features could help differentiate HER2-zero, HER2-low, and HER2-positive tumors. They tested their method on women with breast cancer who underwent MRI at two different centers between 2020 and 2022. The team also performed tumor segmentation and radiomic feature extraction on T2-weighted and dynamic contrast-enhanced T1-weighted images.
The team extracted a total of 101 features from 2D T2-weighted images and 107 features from 3D T1-weighted images. After unsupervised correlation analysis, 31 features were entered into the feature selection model. This included 14 T2-weighted and 17 dynamic contrast-enhanced T1-weighted images. The team obtained a radiomic signature using logistic regression of the top seven least absolute shrinkage and selection operator (LASSO)-selected features. This separated HER2-low and HER2-positive cancers from HER2-zero cancers.
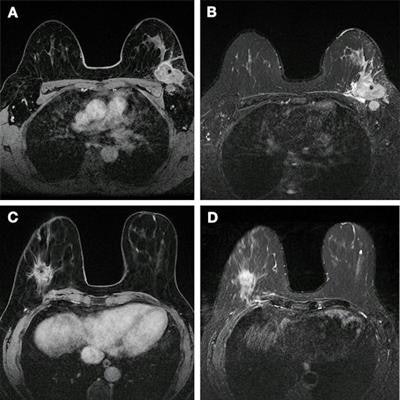
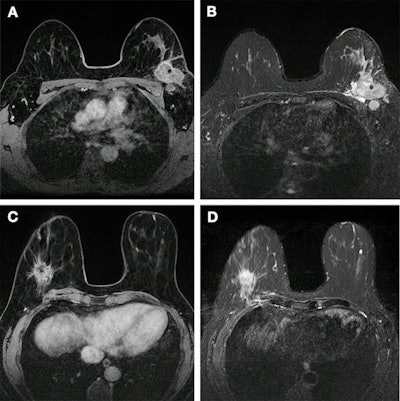 Images show breast cancer in two women at baseline multiparametric MRI. (A) Image shows axial delayed-phase dynamic contrast-enhanced MRI scan in a 51-year-old woman with luminal B no special type carcinoma in the left breast 240 seconds after gadolinium chelate injection. (B) Image shows axial T2-weighted image in the same woman as in (A). At core biopsy, the lesion was considered as HER2 low. The lesion was correctly classified by the radiomic signature (0.87 ≥ threshold). (C) Image shows axial delayed-phase dynamic contrast-enhanced MRI scan in a 43-year-old woman with luminal B no special type carcinoma in the right breast 240 seconds after gadolinium chelate injection. (D) Image shows axial T2-weighted image in the same woman as in (C). At core biopsy, the lesion was considered as HER2 zero. The lesion was correctly classified by the radiomic signature (0.45 < threshold). Images and caption courtesy of the RSNA.
Images show breast cancer in two women at baseline multiparametric MRI. (A) Image shows axial delayed-phase dynamic contrast-enhanced MRI scan in a 51-year-old woman with luminal B no special type carcinoma in the left breast 240 seconds after gadolinium chelate injection. (B) Image shows axial T2-weighted image in the same woman as in (A). At core biopsy, the lesion was considered as HER2 low. The lesion was correctly classified by the radiomic signature (0.87 ≥ threshold). (C) Image shows axial delayed-phase dynamic contrast-enhanced MRI scan in a 43-year-old woman with luminal B no special type carcinoma in the right breast 240 seconds after gadolinium chelate injection. (D) Image shows axial T2-weighted image in the same woman as in (C). At core biopsy, the lesion was considered as HER2 zero. The lesion was correctly classified by the radiomic signature (0.45 < threshold). Images and caption courtesy of the RSNA.The training set included 208 women with an average age of 53 years from one center, while the external test set included 131 patients with an average age of 54 years from a second center.
The researchers found that the radiomic signature achieved an area under the curve (AUC) of 0.8 in the external test set for differentiating HER2-low and HER2-positive tumors from HER2-zero tumors. They also reported that the radiomic signature was a significant predictive factor for distinguishing these two groups, with an odds ratio of 7.6 (p < 0.001).
The team also found that combining histology type, associated non-mass enhancement, and multiple lesions at MRI achieved an AUC of 0.77 in the external test set for the prediction of HER2-positive versus HER2-low cancers.
The study authors suggested that pathologists could be alerted to potential HER2 expression in breast tumors by integrating the team's radiomic signature into their workflow as a support diagnosis tool. This would make the signature a noninvasive biomarker in evaluating whole-tumor heterogeneity and help select biopsy targets.
"Also, the signature could be easily reassessed to monitor spatiotemporal tumor biology changes at each progression of the disease," the authors wrote.
They also suggested that retesting for HER2-low expression using imaging guidance could be an option for enrollment in ongoing clinical trials of anti-HER2 therapies, "given the limited treatment options for the resistant luminal and triple-negative subpopulations at advanced stages."
The full study can be found here.