Top Story
Latest News
Median posts Q1 results, reports net loss for 2023
April 26, 2024
Esaote reports growth in fiscal year 2023
April 25, 2024
Cases of the Week
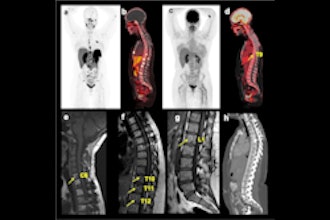
Check out our Cases of the Week!
More from AuntMinnieEurope
Advanced visualization in 2024: Enhancing radiology care
April 25, 2024
Smart Reporting secures €23M in financing
April 25, 2024
EUCAIM calls for new applicants and prepares for webinar
April 25, 2024
First patient dosed with ITM-31 in glioblastoma trial
April 23, 2024
ESR releases movie to mark key moments of ECR 2024
April 23, 2024
Blackford, Annalise.ai enter partnership
April 23, 2024
Anger builds over 10% fee hike for RCR members
April 23, 2024
RCR: Radiologist shortage continues
April 22, 2024