U.K. software developer Ultromics has received U.S. Food and Drug Administration (FDA) clearance for EchoGo Core, the company's artificial intelligence (AI) software for earlier detection of cardiovascular disease.
EchoGo automates the analysis of echocardiography exams, which traditionally have been interpreted by cardiologists. The software uses AI algorithms to calculate left ventricular ejection fraction, left ventricular volumes, and automated cardiac strain.
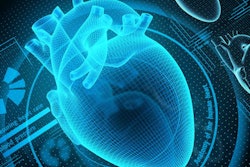
 EchoGo Core is designed to enable the earlier detection of cardiovascular disease. Image courtesy of Ultromics.
EchoGo Core is designed to enable the earlier detection of cardiovascular disease. Image courtesy of Ultromics.Ultromics sees the ability to calculate cardiac strain as a particular selling point for the technology, enabling clinicians to rapidly obtain accurate and repeatable calculations of strain parameters. The calculation of strain will be reimbursable in the U.S. starting in January 2020, the company noted.
Ultromics has partnerships with U.S. clinical centers and 30 National Health Service (NHS) facilities in the U.K. The company is also planning to expand into other regions, such as Europe and Asia.
The firm is a spin-off of Oxford University in the U.K., and it began developing EchoGo in 2011. Ultromics plans to develop EchoGo Pro for cardiac disease prediction in 2020.