This year's annual French radiology congress, Journées Francophone de Radiologie (JFR), is drawing attention to the precautions that must be taken when using gadolinium contrast agents in pregnant and lactating women.
As a precautionary measure, injection of gadolinium chelate should be avoided during pregnancy unless strictly necessary if the desired diagnostic information cannot be obtained from the morphological and functional sequences without injection, noted the authors, Dr. Marianne Alison and colleagues from the University Hospitals of Paris, University of Tours, and Sorbonne University.
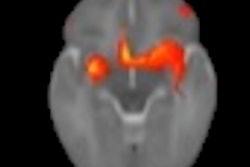
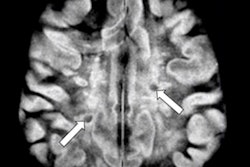
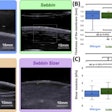
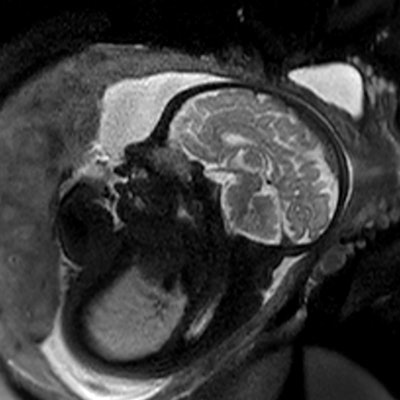
 Technological developments have made it possible to reduce the number of MRI examinations requiring contrast. The injection decision should be based on the principle of justification, depending on the pathologies and the terrain. For pregnant women, the benefit/risk ratio should be assessed on a case-by-case basis. Image courtesy of JFR e-Quotidien.
Technological developments have made it possible to reduce the number of MRI examinations requiring contrast. The injection decision should be based on the principle of justification, depending on the pathologies and the terrain. For pregnant women, the benefit/risk ratio should be assessed on a case-by-case basis. Image courtesy of JFR e-Quotidien.The team noted that when it was unavoidable, doctors must use only the smallest dose necessary and the most stable (macrocyclic) contrast agents. The injection of gadolinium chelate should not be systematic in cases of abnormal placental insertion but instead reserved for complex cases.
In the event of an injection during the first trimester, a declaration to the Reference Center for teratogenic agents (CRAT) is desirable: www.lecrat.fr. If pregnancy is discovered after injection of gadolinium-based contrast agents, the patient should however be reassured. In a lactating woman, the most stable contrast agents (macrocyclic) are to be preferred. It is not necessary to stop breastfeeding after an injection of gadolinium chelate, the authors noted.
Few clinical data exist on the use of gadolinium contrast media during pregnancy or while breastfeeding. In existing data from animal studies, the team revealed that only a very small amount of gadolinium crosses the placental barrier (<0.001% in macaques) and that in a 2016 single retrospective cohort study in a group of fetuses exposed to gadolinium chelate in utero, there was no teratogenic effect.
Despite these data, the precautionary principle must prevail, due to a lack of data on the kinetics of decrease of gadolinium in the tissues of the human fetus, in particular in the kidneys, liver, and skin, and a lack of data on the possible deposition of gadolinium in brain tissue, as well as on the possible dechelation of gadolinium chelates, including the more stable (macrocyclic) in amniotic fluid.
Editor's note: This is an edited version of a translation of an article published in French online by the daily newspaper, e-Quotidien, of Journées Francophones de Radiologie (JFR). Translation by Frances Rylands-Monk. To read the original version, go to the JFR website.