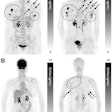
Following decades of development in particle physics labs, the use of beams of protons or ions to kill tumors is beginning to transform the global cancer therapy scene.
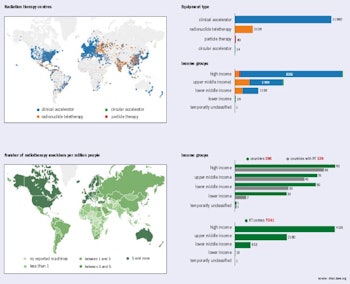
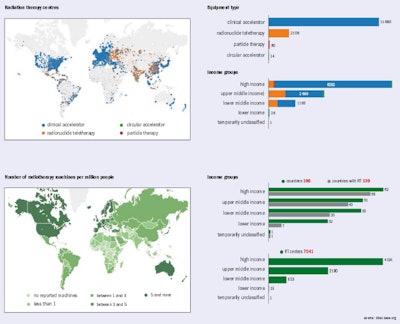
Statistics at a glance. Image courtesy of dirac.iaea.org.
Cancer is a critical societal issue. Worldwide, in 2012 alone, 14.1 million cases were diagnosed, 8.2 million people died, and 32.5 million people were living with cancer. These numbers are projected to rise by 2030 to reach 24.6 million newly diagnosed patients and projected deaths of 13 million. While the rate of cancer diagnoses is growing only steadily in the most developed countries, less developed countries can expect a two-fold increase in the next 20 years or so. The growing economic burden imposed by cancer -- amounting to around $2 trillion U.S. (1.63 trillion euros) worldwide in 2010 -- is putting considerable pressure on public healthcare budgets.
Radiotherapy, in which ionizing radiation is used to control or kill malignant cells, is a fundamental component of effective cancer treatment. It is estimated that about half of cancer patients would benefit from radiotherapy for treatment of localized disease, local control, and palliation. The projected rise in cancer cases will place increased demand on already scarce radiotherapy services worldwide, particularly in less developed countries.
In 2013, member states of the World Health Organization agreed to develop a global monitoring framework for comprehensive, noncommunicable diseases (NCDs). The aim is to reduce premature mortality from cardiovascular and chronic respiratory diseases, cancers, and diabetes by 25%, relative to 2010 levels, which means 1.5 million deaths from cancer will need to be prevented each year.
Advanced cancer therapy techniques based on beams of protons or ions are among several tools that are expected to play a significant role in this effort. In addition, advanced imaging and detection technologies for high-energy physics research -- many being driven by CERN and the physics community -- are needed. These include in-beam PET and prompt-gamma imaging, and treatment planning based on the latest Monte Carlo simulation codes.
Optimal dose
The main goal of radiotherapy is to maximize the damage to the tumor while minimizing the damage to the surrounding healthy tissue, thereby reducing acute and late side effects. The most frequently used radiotherapy modalities use high-energy (MeV) photon or electron beams. Conventional x-ray radiation therapy is characterized by almost exponential attenuation and absorption, delivering the maximum energy near the beam entrance, but continuing to deposit significant energy at distances beyond the cancer target.
The maximum energy deposition for x-ray beams with energy of about 8 MeV is reached at a depth of 2 cm to 3 cm in soft tissue. To deliver dose optimally to the tumor, while protecting surrounding healthy tissues, radiotherapy has progressed rapidly with the development of new technologies and methodologies. The latest developments include MRI-guided radiotherapy, which combines simultaneous use of MRI and photon irradiation. Such advanced radiation therapy modalities are becoming increasingly important and offer new opportunities to treat different cancers, in particular the combination with other emerging areas such as cancer-immunotherapy and the integration of sequencing data, with clinical decision-support systems for personalized medicine.
 Figure 1: For protons and other ions the peak of energy loss occurs just before the particles reach a halt. This is called the Bragg peak, first put to use at Lawrence Berkeley Laboratory to treat the first patient with protons in 1954.
Figure 1: For protons and other ions the peak of energy loss occurs just before the particles reach a halt. This is called the Bragg peak, first put to use at Lawrence Berkeley Laboratory to treat the first patient with protons in 1954.However, if one looks at the dose deposition profile of photons compared with other particles (figure 1), the conspicuous feature of this graph is that, in the case of protons and carbon ions, a significant fraction of the energy is deposited in a narrow depth range near the endpoint of the trajectory, after which very little energy is deposited. It was precisely these differences in dose -- the so-called Bragg-peak effect -- that led visionary physicist and founder of Fermilab, Robert Wilson, to propose the use of hadrons for cancer treatment in 1946.
Several advantages
Hadron or particle therapy is a precise form of radiotherapy that uses charged particles instead of x-rays to deliver a dose of radiotherapy to patients. Radiation therapy with hadrons or particles (protons and other light ions) offers several advantages over x-rays: Not only do hadrons and particles deposit most of their energy at the end of their range, but particle beams can be shaped with great precision. This allows for more accurate treatment of the tumor, destroying the cancer cells more precisely with minimal damage to surrounding tissue. Radiotherapy using the unique physical and radiobiological properties of charged hadrons also allows highly conformal treatment of various kinds of tumors, in particular those that are radio-resistant.
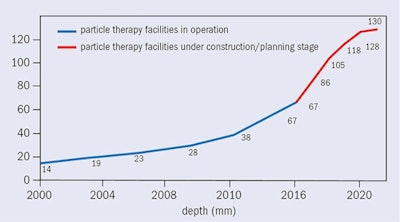 Figure 2: Hadron therapy facilities in operation worldwide, under construction and in the planning stage, at the end of 2016.
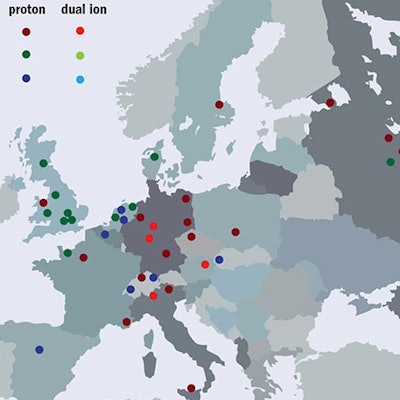
Figure 2: Hadron therapy facilities in operation worldwide, under construction and in the planning stage, at the end of 2016.Over the past two decades, particle-beam cancer therapy has gained huge momentum. Many new centers have been built, and many more are under construction (figure 2). At the end of 2016, there were 67 centers in operation worldwide and another 63 are in construction or in the planning stage. Most of these are proton centers: 25 in U.S. (protons only); 19 in Europe (three dual centers); 15 in Japan (four carbon and one dual); three in China (one carbon and one dual); and four in other parts of the world. By 2021 there will be 130 centers operating in nearly 30 countries. European centers are shown in figure 3 while figure 4 shows the cumulated number of treated patients is growing almost exponentially.
At the end of 2007, 61,855 patients had been treated (53,818 with protons and 4,450 with carbon ions). At the end of 2016, the number had grown to 168,000 (145,000 with protons and 23,000 with carbon ions). This is due primarily to the greater availability of dedicated centers able to meet the growing demand for this particular form of radiotherapy, and most probably in future it will have a larger growth rate, with an increase of the patient throughput per center.
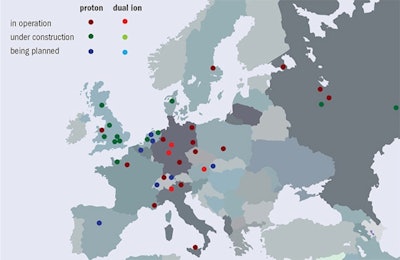 Figure 3: European hadron therapy facilities in operation or under construction in 2016.
Figure 3: European hadron therapy facilities in operation or under construction in 2016.Particle-physics foundation
High-energy physics research has played a major role in initiating, and now expanding, the use of particle therapy. The first patient was treated at Berkeley National Laboratory in the U.S. with hadrons in September 1954 -- the same year CERN was founded -- and was made possible by the invention of the cyclotron by Ernest Lawrence and subsequent collaboration with his medical-doctor brother, John. The first hospital-based, particle-therapy centers opened in 1989 at Clatterbridge in the U.K. and in 1990 at the Loma Linda University Medical Center in the U.S. Before this time, all research related to hadron therapy and patient treatment was carried out in particle-physics labs.
In addition to the technologies and research facilities coming from the physics community, the culture of collaboration at the heart of organizations such as CERN is finding its way into other fields. This has inspired the European Network for Light Ion Therapy (ENLIGHT) to promote international discussions and collaboration in the multidisciplinary field of hadron therapy, which has now been running for 15 years.
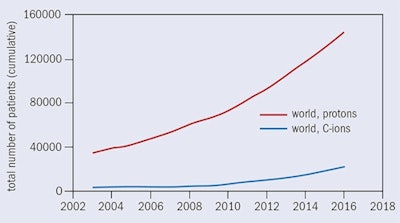 Figure 4: Patients treated with protons and carbon ions worldwide by the end of 2016.
Figure 4: Patients treated with protons and carbon ions worldwide by the end of 2016.Were it not for the prohibitively large cost of installing proton-therapy treatment in hospitals, it would be the treatment of choice for most patients with localized tumors. Proton-therapy technology is significantly more compact today than it once was, but when combined with the gantry and other necessary equipment, even the most compact systems on the market occupy an area of a couple of hundred square meters. Most hospitals lack the financial resources and space to construct a special building for proton therapy, so we need to make facilities smaller and cheaper, with costs of around $5 million to 10 million U.S. (4 million to 8 million euros) for a single room, similar to state-of-the-art photon-therapy systems.
An aging population, and the need for a more patient-specific approach to cancer treatment and other age-related diseases, present major challenges for future technologies to control rising health costs, while continuing to deliver better outcomes for patients. Scientists working at the frontiers of particle physics have much to contribute to these goals, and the culture of collaboration will ensure that breakthrough technologies find their way into the medical clinics of the future.
This article first appeared in the January/February 2018 issue of CERN Courier. Republished with permission from CERN, which holds copyright.