EuroMinnies finalists, page 2
Scientific Paper of the Year
Mortality due to cancer treatment delay: Systematic review and meta-analysis. Hanna T et al, BMJ, 4 November 2020.
This 4 November BMJ study proved fears about the consequences of delayed cancer diagnosis due to the COVID-19 pandemic may be well-founded. In the study, a team of Canadian and U.K. authors found a patient whose cancer treatment is delayed by even one month can face a mortality risk of up to 13% higher.
"There is a growing realization that particularly national or regional lockdowns, come at a cost to wider health and welfare of societies," stated the authors, led by Dr. Timothy Hanna, PhD, clinician-scientist at Queen's University in Kingston, Canada. "We now need to count the previously invisible cost of COVID-19 on people with cancer."
The research team evaluated seven major cancer types (bladder, breast, colon, rectum, lung, cervix, and head and neck) and three types of treatments (surgery, systematic treatments such as chemotherapy, and radiotherapy). Their main objective was to measure the risk to overall survival per four-week delay from diagnosis to first treatment, or from the completion of one treatment to the start of the next.
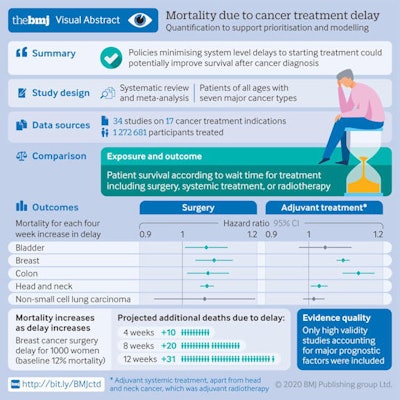 Graphic shows the main results of the study. Courtesy of Hanna et al. BMJ open access.
Graphic shows the main results of the study. Courtesy of Hanna et al. BMJ open access.A treatment delay of four weeks was associated with an increase in the risk of death for all three treatment types, and the risk of death was greater for longer delays. For example, for breast cancer surgery, the risk of death would increase by 17% for an eight-week delay and 26% for a 12-week delay.
"These results are sobering and suggest that the survival gained by minimizing the time to initiation of treatment is of similar (and perhaps greater) magnitude of benefit as that seen with some novel therapeutic agents," the authors noted.
The study was limited by relying on observational research that cannot establish causation. The results also did not consider the impact of treatment delay on factors other than death, such as functional outcomes, complications from more extensive treatments, quality of life, or the greater economic burden.
To read the full article, go to the BMJ website.
The use of imaging in COVID-19 -- results of a global survey by the International Society of Radiology. Blažić I et al, European Radiology, 17 September 2020.
This global survey of 50 radiology departments highlighted the significant variations in imaging procedures for patients with COVID-19. Facilities from 33 countries reported relying on different modalities for imaging patients with varying disease severity in the 17 September publication in European Radiology.
"All imaging departments involved in this survey reported the use of imaging in COVID-19 patients showing severe symptoms or who were critically ill," noted first author Dr. Ivana Blažić, PhD, MRI section head in Clinical Hospital Center Zemun in Belgrade, Serbia, and colleagues. "However, there is a wide variation in imaging modality type used for each clinical scenario."
While 100% of surveyed departments said they performed imaging in patients with critical illness, the modalities they employed were far from uniform:
- 33% used chest x-ray (CXR).
- 19% used chest CT.
- 23% used both CXR and CT.
- 13% of departments used CXR, CT, and lung ultrasound.
Facilities reported using similarly varied modalities for imaging performed in patients with severe symptoms as well as those with confirmed or suspected cases of COVID-19.
The institutions were also split on whether they used imaging at the end of the confinement (60% said yes) or in asymptomatic patients (69% said no). However, the majority of centers said the COVID-19 pandemic affected the radiology's department's routine activity.
The authors hoped the survey would increase the understanding of heterogeneities in radiology practice and to identify needs and gaps of radiology departments in relation to the COVID-19 pandemic. They also presented a summary of the recommendations for professional societies from countries particularly large COVID-19 outbreaks.
"The guidelines on the use of imaging in COVID-19 differ at a smaller or larger extent depending on the country or region and the level of and access to healthcare in the respective country or area of the world and it seems that they also reflect the local situation of the COVID-19 pandemic," they noted.
To read the full article, go to the European Radiology website.
Best New Radiology Device
Aquilion Exceed LB CT scanner, Canon Medical Systems
Medical imaging is playing an increasingly prominent role in planning and guiding radiation therapy procedures. Our first finalist in the Best New Radiology Device category -- Aquilion Exceed LB -- reflects this new emphasis as a CT scanner with an ultrawide 90-cm bore that was designed from the ground up for use in radiation oncology.
When it comes to radiation oncology, CT vendors typically repurpose existing scanners designed for diagnostic use. But Canon Medical Systems decided to take a different tack, going back to the drawing board to design a system purpose-built for planning radiation therapy.
To start with, Canon set the width of Aquilion Exceed LB's bore at 90 cm, which the company believes is the widest in the industry and better meets the unique patient positioning needs in radiation therapy. The system uses a 160-slice CT scanner with 4 cm of coverage per rotation.
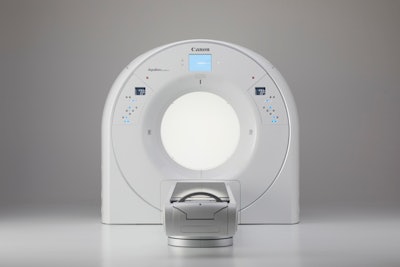 Canon's new Aquilion Exceed LB scanner. Image courtesy of Canon.
Canon's new Aquilion Exceed LB scanner. Image courtesy of Canon.What's more, data acquired with Aquilion Exceed LB are reconstructed across the entirety of the 90-cm field-of-view, and the system also uses Canon's Advanced intelligent Clear-IQ Engine (AiCE) Deep Learning Reconstruction (DLR) technology, pioneered on the vendor's diagnostic radiology CT line.
The scanner includes features such as one-touch 3D isocentering to enable faster image acquisition, while AiCE reconstruction enables the creation of more accurate contouring -- another important consideration in radiation therapy planning. The device's table can also be moved laterally 8.5 cm in either direction for easier patient positioning.
Canon first launched Aquilion Exceed LB at the 2020 American Society for Radiation Oncology (ASTRO) virtual meeting in October. More information about Aquilion Exceed LB is available here.
Magnetom Free.Max wide-bore MRI unit, Siemens Healthineers
Over the decades, MRI has gravitated toward ever-more-powerful magnets, first 1.5-tesla and then 3-tesla, and now even 7-tesla systems are being pondered for clinical use. But Siemens Healthineers moved in the other direction with Magnetom Free.Max, a 0.55-tesla scanner that is the other finalist for Best New Radiology Device in the EuroMinnies.
Introduced in November 2020, Magnetom Free.Max is the smallest and most lightweight whole-body MRI scanner that Siemens Healthineers has ever commercialized. The system is designed to open up new clinical applications to users while also offering excellent economic value and low total cost of ownership.
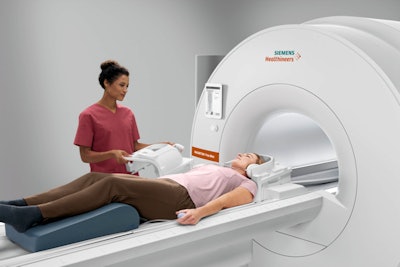 Magnetom Free.Max. Image courtesy of Siemens Healthineers.
Magnetom Free.Max. Image courtesy of Siemens Healthineers.The scanner's origin story comes from the belief at Siemens Healthineers that despite MRI's great success, the modality is still underutilized. Hospitals may not want to site a magnet a great distance from the radiology department, such as in the accident and emergency department, instead preferring CT. Other facilities, like imaging centers, might balk at the high price tag of 1.5-tesla scanners.
Instead, Magnetom Free.Max is affordable enough to make MRI accessible to a new class of customers, while also being small enough to install in a room in the intensive care unit. It also has an 80-cm bore, which makes it more suitable for scanning obese patients.
Helium refills are a hassle for any user of a superconducting MRI scanner. But Siemens Healthineers addressed this pain point by designing Magnetom Free.Max to use less than 1 liter of helium to keep its superconducting magnet cold. What's more, the scanner doesn't need a quench pipe, which adds up to further savings on installation costs.
Magnetom Free.Max will be a global product for the company, with initial deliveries beginning in Europe. More information about Magnetom Free.Max is available here.
Best New Radiology Software
BerlinCaseViewer app for COVID-19 case recognition, BerlinFlame GmbH
As the pandemic spread throughout the world in 2020, radiologists had to quickly learn how to recognize the disease's typical radiological signs. To help meet this urgent challenge, the developers of this training app shifted from their usual focus on imaging of rheumatic diseases to create an educational resource specifically for COVID-19.
The free app now also provides users with full datasets of helpful COVID-19 and non-COVID-19 cases in an interactive, case-based quiz format. Having access to the entire CT dataset is important, as radiologists need to learn to pick the slice that best shows the pathology in question, or the relevant radiological signs, according to app founder Dr. Kay-Geert Hermann, consultant radiologist at the Charité in Berlin.
Radiologists can access BerlinCaseViewer for free at berlincaseviewer.de or directly in the Apple App Store. Once downloaded, the app will present the users with relevant cases, the patient's clinical history, and the chance to scroll through the entire CT dataset for that patient. Each dataset typically contains over 50 slices per case.
 Users can scroll through the full CT dataset per case and access the clinical history available before deciding on diagnosis. Image courtesy of Dr. Kay-Geert Hermann.
Users can scroll through the full CT dataset per case and access the clinical history available before deciding on diagnosis. Image courtesy of Dr. Kay-Geert Hermann.Of the cases, half are COVID-19 patients and half are non-COVID-19 cases, including a range of pneumonia types and pathologies that can mimic COVID-19 or are in differential diagnosis with it. A "lighter" version of the app with fewer cases is also available for radiologists in countries with limited internet access or slower bandwidth.
Once the user has made a diagnosis, the app will display colored overlays showing the radiological signs typical of the relevant pathology, according to Hermann.
For more details, see the AuntMinnieEurope.com report from 29 October.
IntelliSpace Portal 12 for AI-assisted quantitative assessment, Philips Healthcare.
Launched at RSNA 2020, the latest version of Philips Healthcare's IntelliSpace Portal Advanced Visualization Workspace features new artificial intelligence (AI)-assisted tools for quantitative assessment and automatic results generation, as well as follow-up and communication with referring physicians.
IntelliSpace Portal 12 includes AI algorithms for detecting lung nodules, analyzing cardiac function, and quantifying pulmonary infiltrates that might be associated with COVID-19. It also brings new applications geared for cardiology, pulmonology, and oncology.
New cardiology tools include, for example, AI-aided segmentation of the right and left ventricles to facilitate volumetric and functional measurements. As a result, a cardiac MRI scan can be completed in under five minutes, Philips said.
In pulmonology, the company's CT Pulmo Auto Results software automatically segments lungs and lesions, as well as classifies ground-glass opacities/consolidation. It also automatically generates reports that include volume summaries and lesion distribution data, Philips said. Additionally, IntelliSpace Portal 12 incorporates Riverain Technologies' CT ClearRead AI application for lung detection and characterization of lung nodules.
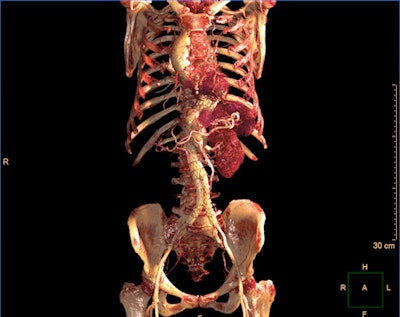 IntelliSpace Portal 12 includes a photorealistic volume-rendering mode. Image courtesy of Philips Healthcare.
IntelliSpace Portal 12 includes a photorealistic volume-rendering mode. Image courtesy of Philips Healthcare.What's more, IntelliSpace Portal 12 includes a new photorealistic volume-rendering technology designed to improve how image volumes are presented in terms of their depth and relationship to other key anatomies, according to the vendor.
Philips has also added support for automatic brain perfusion measurements as part of IntelliSpace Portal 12's CT brain perfusion analysis package. In addition to being instantly available in PACS, these measurements can also be automatically emailed to clinicians, the company said.
Read more in this AuntMinnie.com report from 3 December.
Best New Radiology Vendor
DeepSpin, Berlin, Germany
This startup firm is developing a low-cost, portable MRI scanner that will leverage the power of artificial intelligence (AI) to offer what the company calls "the first truly low-cost MRI machine."
The firm believes its technology will be a fraction of the cost, size, and weight of conventional MRI scanners. It plans to do this by basing its scanners on simplified hardware using a new type of coil and magnet setup, with advanced AI algorithms used to analyze and process images. The company envisions its scanners weighing 200 kg, instead of many tons as conventional scanners do.
 Managing directors Pedro Freire Silva (left), an MRI engineer specializing in hardware and applications who was a PhD researcher at Karlsruhe Institute of Technology in Germany, and Clemens Tepel (right), a former consultant in strategy and operations at McKinsey, and Photo courtesy of DeepSpin.
Managing directors Pedro Freire Silva (left), an MRI engineer specializing in hardware and applications who was a PhD researcher at Karlsruhe Institute of Technology in Germany, and Clemens Tepel (right), a former consultant in strategy and operations at McKinsey, and Photo courtesy of DeepSpin.DeepSpin believes that the low cost and portability of its technology will enable MRI scanners to be employed in many areas that have been unreachable until now, either due to the modality's cost or difficulty in siting. Developing countries or physician offices could be ready markets for DeepSpin's technology.
The company has already begun winning design and technology awards, and in mid-2020 won a seed round of financing to further develop its technology.
ImageBiopsy Lab, Vienna, Austria
ImageBiopsy Lab is developing a range of artificial intelligence (AI)-based software applications to assist in the diagnosis of musculoskeletal (MSK) diseases, such as knee osteoarthritis, leg-length discrepancy, and femoroacetabular impingement.
The company notes that MSK diagnoses are often inconsistent and subjective, while MSK diseases are prevalent, with over 1 billion people globally suffering from orthopedic diseases. At the same time, early diagnosis can lead to treatment and prevent unnecessary surgeries.
 CEO and co-founder Richard Ljuhar. Previously he was director of research and development for Musculoskeletal Health Products, Braincon, and a researcher at the Technical University of Vienna. Photo courtesy of ImageBiopsy Lab.
CEO and co-founder Richard Ljuhar. Previously he was director of research and development for Musculoskeletal Health Products, Braincon, and a researcher at the Technical University of Vienna. Photo courtesy of ImageBiopsy Lab.Conventional radiography is most frequently used as the first imaging modality for MSK conditions, but the volume of radiographs acquired can create cost and workflow challenges for radiology departments.
ImageBiopsy Lab hopes to solve the problem with software applications that it believes can save up to three minutes of interpretation time per radiograph. This can add up to 45-60 extra minutes per day in time savings for a radiologist.
The company's products carry whimsical names like KOALA (knee osteoarthritis labeling assistant), PANDA (pediatric skeletal maturity assessment and growth potential estimation), and HIPPO (femoroacetabular impingement and hip dysplasia).
In November 2020, the company got a major boost by signing a deal with multimodality vendor GE Healthcare in which ImageBiopsy Lab's software will become a part of the Edison AI ecosystem that GE is building. Findings from ImageBiopsy Lab applications will be available to users of GE PACS and universal viewer software through the company's Edison Open AI Orchestrator link.
ImageBiopsy Lab also signed a deal with Siemens Healthineers in July 2020 in which its KOALA software will be made available to Siemens customers.