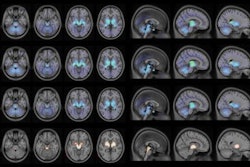
A plasma tau biomarker and tau PET imaging performed equally well at identifying Alzheimer's disease pathology, yet tau PET appears superior for monitoring disease staging and clinical progression, according to a group in the Netherlands.
Researchers at Vrije Universiteit Amsterdam UMC performed a head-to-head comparison of plasma p-tau181 and tau PET for identifying Alzheimer's disease (AD) pathology and predicting clinical progression in patients at different stages of disease. Tau PET had an edge in detecting cognitive changes over time, they found.
"With the recent FDA approval of the tau PET tracer F-18 flortaucipir for clinical use, and intentions for [p-tau181] to eventually be used in the clinic, there is a need to compare these biomarkers to guide clinicians," wrote corresponding author Emma Coomans, a radiology and nuclear medicine doctoral candidate, and colleagues. (14 October, Journal of Nuclear Medicine).
Neurofibrillary tau tangles consist of hyperphosphorylated tau (p-tau) and are a pathological hallmark of Alzheimer's disease (AD). Tau pathology in AD is closely associated with clinical symptoms and disease severity. Thus, in vivo assessments of tau provide both accurate diagnostic and prognostic information.
Two biomarkers for detecting in vivo tau pathology associated with AD include p-tau181 measurements in cerebrospinal fluid and radiotracer binding in brain tau tangles on PET.
Both biomarkers have proven useful in clinical trials. Blood-based biomarkers have major advantages, including easy accessibility, wide applicability, relative noninvasiveness, and low costs, and can therefore easily be repeated over time. Conversely, PET biomarkers, although expensive, have the advantage of providing spatial information of tracer binding throughout the brain, the authors wrote.
In this study, the researchers analyzed tests in patients from a previous study who underwent F-18 flortaucipir (Tauvid, Avid Radiopharmaceuticals) PET and had a plasma sample biobanked within 12 months. They included 50 patients with subjective cognitive decline (SCD) and 60 participants with mild cognitive impairment or dementia due to AD (MCI/AD). A subset of 40 patients had two-year longitudinal p-tau181 and tau PET available, with test data covering a mean 3.2 years.
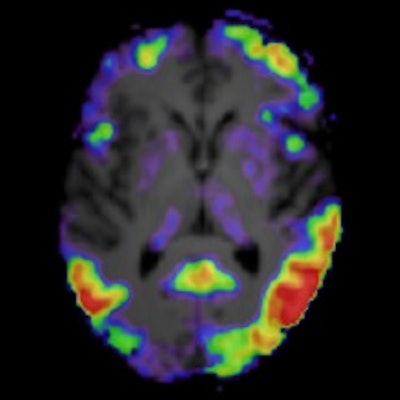
 A graphical abstract. Image courtesy of the Journal of Nuclear Medicine.
A graphical abstract. Image courtesy of the Journal of Nuclear Medicine.Plasma p-tau181 and tau PET were compared in their accuracies in discriminating between cognitive stage (MCI/AD vs. SCD) and preclinical status, their associations with cross-sectional and longitudinal neuropsychological test performance, and their longitudinal changes over time.
According to the analysis, when discriminating between preclinical status, the area under the curve (AUC) for plasma p-tau181 was 0.83, while tau PET AUCs were equally high (entorhinal, 0.87; temporal, 0.85; neocortical, 0.67).
However, the researchers found that tau PET outperformed plasma p-tau181 in discriminating MCI/AD from SCD. In this regard, p-tau181 achieved an AUC of 0.74, while tau PET AUCs in specific brain regions were as follows: entorhinal, 0.89; temporal, 0.92; and neocortical, 0.89 (all p < 0.01).
"Overall, tau PET showed stronger associations with cognitive decline and was associated with a wider variety of cognitive tests compared to plasma p-tau181," the authors wrote.
In addition, while both plasma p-tau181 and tau-PET increased more steeply over time in MCI/AD compared with SCD (p < 0.05), only tau-PET annual changes were associated with cognitive decline, the authors added.
Ultimately, the findings may have implications for how researchers design clinical trials. The study supports both plasma p-tau181 and tau PET as biomarkers for identifying AD pathology, yet advantages may be gained by using a relatively simple plasma test to enroll patients in trials, while reserving more expensive PET scans for evaluating patient response to new treatments, the authors suggested.
"Although our longitudinal results should be cautiously interpreted due to small sample size, our finding warrants further investigation as it could have implications for clinical trial designs," they wrote.