An artificial intelligence (AI) algorithm was able to produce high-quality whole-body diffusion-weighted MRI (WBDWI) in a fraction of the time traditionally needed to perform these exams, potentially enabling wider use of clinical WBDWI, U.K. researchers reported at RSNA 2019.
A team of researchers led by Konstantinos Zormpas-Petridis, a doctoral student at the Institute of Cancer Research (ICR) in London, found that an AI algorithm could generate clinical-grade images from subsampled data, potentially reducing acquisition times from approximately 30 minutes to about five minutes.
"Such time-savings could reduce scanning costs," as well as render WBDWI appropriate for screening studies and reduce imaging time and discomfort for patients, Zormpas-Petridis told AuntMinnieEurope.com.
An attractive noninvasive tool
 Konstantinos Zormpas-Petridis, a doctoral student at the Institute of Cancer Research.
Konstantinos Zormpas-Petridis, a doctoral student at the Institute of Cancer Research.WBDWI is an attractive noninvasive tool for staging and evaluating treatment response in patients with lymphoma and metastatic bone disease from prostate and breast cancers. Bone is the most common site of metastasis in prostate cancer; more than 50% of patients have only bone metastases, according to Zormpas-Petridis, who works within the computational imaging team at the ICR, led by Matthew Blackledge, PhD. These bone metastases cause pain, skeletal fracture, and spinal cord compression.
In addition, guidelines from the U.K.'s National Institute for Health and Care Excellence (NICE) "consider whole-body MRI as first-line imaging" for suspected or newly diagnosed myeloma, he said.
The development of new treatments for bone metastases, however, is hindered by the lack of robust response criteria in assessing benefit from therapy, he noted.
A 'calamitous bottleneck'
One issue is that WBDWI requires a total study time of approximately one hour, including patient setup and positioning. Thirty minutes of DWI is performed, along with 15 minutes of T1-weighted and T2-weighted imaging. WBDWI requires nine to 12 excitations for each slice to overcome the inherently low signal-to-noise ratio of the technique, resulting in long acquisition times, according to the researchers.
"This provides a calamitous bottleneck for the adoption of WBDWI into a general clinical workflow; an ever-increasing patient population increases capacity pressures on imaging centers, which are then less willing to perform such long imaging studies," Zormpas-Petridis said. "Furthermore, patients for which WBDWI has shown to be clinically useful are typically frail and have painful conditions, which lowers patient acceptance of the methodology."
As a result, accelerated acquisition schemes are highly sought after, he said. The researchers therefore sought to utilize a deep-learning network to determine whether subsampled -- but rapidly acquired -- images could be used to reconstruct high-quality images.
They created a training set of 59,400 total images by using 160 to 200 slices each for nine acquisitions from 14 patients. After this dataset was used to train a U-Net-based regression model, the researchers evaluated the quality of images that were generated by the AI algorithm from analysis of just the initial WBDWI acquisition.
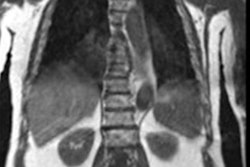
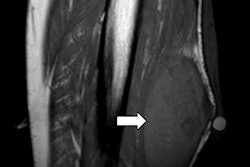
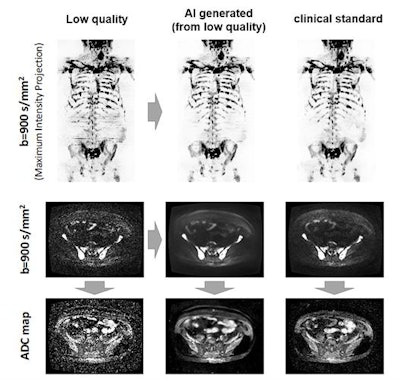 The AI algorithm generates whole-body diffusion-weighted MR images (middle) from subsampled/low-quality (left) but rapidly acquired images. The model generates images of comparable diagnostic quality to the clinical standard (right) and can be used for assessing metastatic disease across the skeleton. Also, apparent diffusion coefficient (ADC) images (a surrogate of tumor cellularity) can be robustly calculated from the AI-generated images, providing a biomarker of treatment response with an increase in ADC values reflecting cell kill. Images courtesy of Konstantinos Zormpas-Petridis.
The AI algorithm generates whole-body diffusion-weighted MR images (middle) from subsampled/low-quality (left) but rapidly acquired images. The model generates images of comparable diagnostic quality to the clinical standard (right) and can be used for assessing metastatic disease across the skeleton. Also, apparent diffusion coefficient (ADC) images (a surrogate of tumor cellularity) can be robustly calculated from the AI-generated images, providing a biomarker of treatment response with an increase in ADC values reflecting cell kill. Images courtesy of Konstantinos Zormpas-Petridis.Better image quality
Using a Likert scale (1 = poor, 5 = excellent), an expert radiologist assessed the image quality on an independent test set of three test patients. The radiologist blindly rated the images from the initial WBDWI acquisition, the arithmetically averaged standard clinical images from the nine total WBDWI acquisitions, and the images generated by the AI algorithm from the initial WBDWI acquisition.
The three types of images were scored for five different parameters: contrast-to-noise ratio, signal-to-noise ratio, tumor detection, image artifacts, and overall image quality. The AI-generated images scored the best for all parameters, yielding an average of 3.7. The images from the initial acquisition had an average score of 1.6, while the averaged standard clinical images from the nine acquisitions had an average score of 2.5.
"Our AI technique provides next-generation image filters that reduce the noise in DWI without reducing image sharpness and, therefore, allow faster acquisition," Zormpas-Petridis said.
The researchers also compared the distribution of apparent diffusion coefficient (ADC) values within segmented bone disease for the three different image types.
"Estimates of mean ADC within bone disease calculated from AI-generated images may be robust for monitoring treatment response," he added.
Prospective validation
Next, the investigators performed a prospective validation of their AI model in 22 patients. They prospectively obtained the initial WBDWI acquisition as well as conventional WBDWI data in the 22 patients. Sensitivity and specificity analysis for disease detection is underway.
The group concluded that the AI approach could provide clinical-grade images from subsampled data, potentially reducing total-body scan times from approximately 30 minutes to about five minutes, Zormpas-Petridis said.
"This approach will translate into the overall acquisition time for a whole-body MRI study to be less than 20 minutes, compared to the 45 to 60 minutes that exists today," he explained.