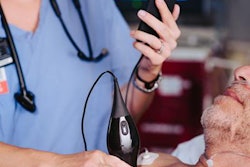
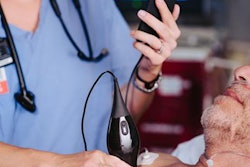
Portable ultrasound developer Butterfly Network has received the CE Mark for its handheld, single-probe, whole-body ultrasound system.
The company plans to begin shipping the Butterfly iQ device in the third quarter to markets that accept the CE Mark.
Since its commercial introduction in 2018, Butterfly iQ has been used by physicians, emergency medical technicians, physician assistants, nurses, and other practitioners in the U.S., according to the company.
The U.S. Food and Drug Administration (FDA) cleared Butterfly iQ in October 2017. It is based on Butterfly Network's Ultrasound-On-Chip technology and is designed to work with iPhones.