Researchers have moved closer to the real-time verification of hadron therapy, demonstrating the in vivo accuracy of simulations that predict particle range in the patient. The new Monte Carlo tool is a key component of a system that measures particle range during treatment and compares it with the predictions.
Housed in the synchrotron facility at the National Centre of Oncological Hadrontherapy (CNAO) in Pavia, the INSIDE system is being developed by an Italian collaboration. It combines a PET scanner that maps positron emitters generated during irradiation and Dose Profiler, a new tracking detector that detects signals from secondary charged particles produced by heavy ion beams.
In their latest study, the researchers used their simulation tool in the first analysis of in vivo PET data, acquired from a single patient in December 2016 (Physica Medica, 7 June 2018).
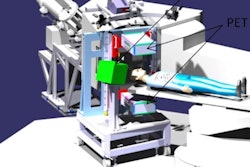
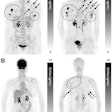
 The INSIDE system positioned for measurement, with detector panels positioned above and below the patient. (Courtesy: E Fiorina et al, Physica Medica, 10.1016/j.ejmp.2018.05.002, © 2018, Associazione Italiana di Fisica Medica)
The INSIDE system positioned for measurement, with detector panels positioned above and below the patient. (Courtesy: E Fiorina et al, Physica Medica, 10.1016/j.ejmp.2018.05.002, © 2018, Associazione Italiana di Fisica Medica)By monitoring particle range during treatment, clinics can identify when changes in anatomy produce unacceptable deviations from a patient's planned treatment. For example, tumors may shrink as they respond to treatment, while specific anatomy such as the paranasal sinuses can contain air or higher-density mucous. Armed with such information, clinicians can better exploit the sharp dose gradients that protons and heavy ions provide to target the tumor and spare healthy tissue.
"An accurate Monte Carlo prediction combined with precision imaging would allow the physician to verify the accuracy of the treatment on a daily basis," said joint first author Elisa Fiorina of the National Institute for Nuclear Physics (INFN) in Turin, Italy. In the longer term, the INSIDE technology could potentially be applied for adaptive particle therapy, in which treatments are modified for changes in patient anatomy.
The simulation tool generates 4D PET scans using the treatment plan parameters, such as beam energies and spot positions. The patient's geometry and composition are provided by the CT scan acquired for treatment planning. Based on these data, the tool predicts the propagation of therapeutic particles in the patient and subsequent generation and annihilation of positron-emitting isotopes. The resulting gamma rays are used to construct the PET scan. The tool incorporates models of the beam line at CNAO, the spatial and temporal characteristics of the treatment beam, as well as the geometry and composition of the INSIDE system.
Preliminary study
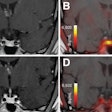
The researchers acquired PET scans during two proton therapy fractions of a 56-year-old patient with carcinoma of the lacrimal gland. Data were acquired over the entire irradiation of one of two fields. Images were updated every 10 sec, enabling a visual, qualitative comparison between the measured and simulated data during treatment.
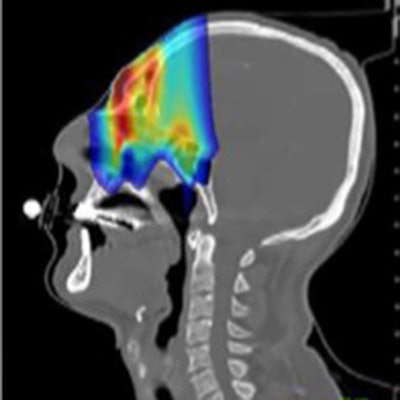
In a comparison with the prescribed treatment plan, dose distributions derived from the simulation proved accurate. Gamma tests demonstrated 91% of voxels agreed to within 3% or 3 mm and 98% of voxels to within 5% or 5 mm.
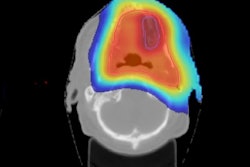
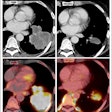
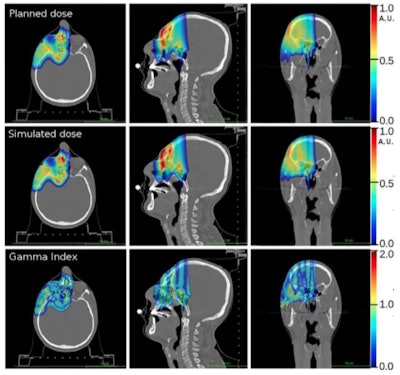 Comparison of prescribed dose distributions calculated by the treatment planning system with dose predicted by the INSIDE Monte Carlo tool. (Courtesy: E Fiorina et al, Physica Medica, 10.1016/j.ejmp.2018.05.002, ©2018, Associazione Italiana di Fisica Medica)
Comparison of prescribed dose distributions calculated by the treatment planning system with dose predicted by the INSIDE Monte Carlo tool. (Courtesy: E Fiorina et al, Physica Medica, 10.1016/j.ejmp.2018.05.002, ©2018, Associazione Italiana di Fisica Medica)Particle range in the predicted and measured PET images was quantified using iso-activity surfaces corresponding to 10% of the maximum voxel intensity in the scans. The researchers demonstrated that the average distance between the surfaces in the two images was less than 1 mm. The analysis was carried out offline, but the authors envisage that a real-time implementation will be straightforward, enabling a quantitative analysis while the patient is still being irradiated.
"These first in vivo measurements demonstrate that the developed Monte Carlo simulation tool ... is accurate enough to be used as a reference in the PET image analysis," Fiorina said.
Based on their findings, the researchers are beginning clinical trials later this year, in which the INSIDE system will incorporate a more precise detector positioning system. The trials will include more rigorous tests of the system's accuracy, including that of the Dose Profiler, using a cohort of around 40 patients. The researchers will also investigate how well the system integrates into routine clinical workflow and potential clinical compliance limits in particle range. The cohort will include individuals with cancers known to respond early to treatment, the group set to benefit most from monitoring.
Jude Dineley is a freelance science writer and former medical physicist based in Bavaria, Germany.
© IOP Publishing Limited. Republished with permission from Physics World, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.