A novel x-ray fluorescence imaging system could enable molecular CT using nonradioactive high-Z tracers, German researchers have found.
Although conventional CT does not provide functional information, recent advances in the imaging of high-Z elements have spurred interest in molecular CT, using gold nanoparticles coupled to antibodies to target particular tumour types, for example.
The most dose-efficient means of detecting high-Z elements is by imaging their x-ray fluorescence. Visualizing the low tracer concentrations relevant to molecular imaging in humans, however, is hindered by large background signals and the need for a large-area detector with high energy resolution. Now, a German research team has proposed a novel imaging scheme that should overcome these problems (Physics in Medicine and Biology, 21 November 2013, Vol. 58:22, pp. 8063-8076).
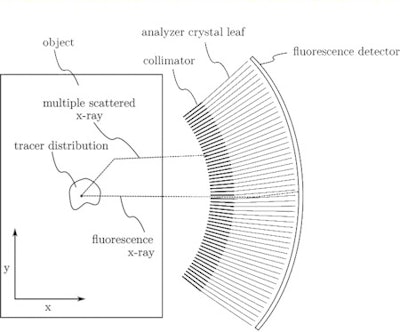 Schematic of the proposed x-ray fluorescence imaging set-up (side-view). All images courtesy of Physics in Medicine and Biology.
Schematic of the proposed x-ray fluorescence imaging set-up (side-view). All images courtesy of Physics in Medicine and Biology."In contrast to existing x-ray fluorescence imaging methods, which are limited by the properties of energy-resolving semiconductor detectors and problematic noise from Compton scattering, our method would largely filter out scattering and provide dose-efficient detection of tracer fluorescence over a large solid angle," explained Bernhard Müller, a PhD student and physicist at Helmholtz Zentrum München. "Ultimately, this could be a step further in the practical application of x-ray fluorescence techniques to clinical molecular imaging, previously only provided by nuclear imaging methods like PET."
The proposed imaging setup uses a high-peak-kilovoltage x-ray tube to provide a filtered pencil beam with a mean energy well above the K-edge of the high-Z tracer. When the beam interacts with the tracer's atoms, characteristic fluorescence x-rays are emitted. These x-rays (together with Compton scattered x-rays) enter a radial collimator and are then directed onto a detector by an array of Bragg reflecting analyser crystal leaves.
Setting the angle of the crystal leaves to the Bragg angle of the tracer's Kα fluorescence means that only the Kα X-rays undergo first-order reflection and reach the detector. Because of the high incident energy of the pencil beam, only multiply scattered x-rays can have energy near that of the tracer fluorescence, but they are mainly nonradially directed and therefore rejected by the collimator.
Proof-of-principle
To demonstrate the feasibility of their proposed setup, Müller and colleagues performed a proof-of-principle experiment examining a fluorescence signal reflected by a single analyser crystal leaf. In this simplified set-up, a filtered 120 kVp x-ray pencil beam was incident upon a 50 µg/ml iodine solution. The emitted x-ray fluorescence passed through a collimator slit (instead of the radial collimator), was reflected by a single crystal leaf, and then recorded by an energy-resolving CdTe detector with a detection area of approximately 7 mm2.
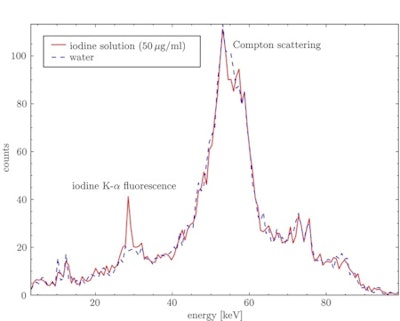 X-ray spectra for a water sample and an iodine solution sample with an iodine concentration of 50 µg/ml. The energy-selective crystal reflection spectrally isolates the Kα iodine fluorescence x-rays from the scattering background.
X-ray spectra for a water sample and an iodine solution sample with an iodine concentration of 50 µg/ml. The energy-selective crystal reflection spectrally isolates the Kα iodine fluorescence x-rays from the scattering background.The observed x-ray spectra contained both the iodine Kα fluorescence peak at about 28.5 keV, in the energy interval corresponding to the crystal's first-order reflection, and a peak at around 55 keV due to second-order reflection of Compton scattered x-rays. While the Compton peak is larger, the iodine fluorescence is spectrally separated by at least 10 keV and should therefore be visible even using a low-energy-resolution detector.
Monte Carlo studies
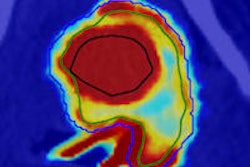
The researchers also modeled the entire imaging setup using the Geant4 Monte Carlo toolkit. They simulated images of a small-animal phantom (a 4 cm diameter "tissue" cylinder containing a 4 mm cylinder filled with iodine solution) by scanning the pencil beam, detector and collimator over the phantom. Recording the contribution of x-rays hitting the detector to each energy bin provides a spectrum at each scan position, from which a projection image of tracer distribution can be generated. To counter Compton scattered x-rays, attenuation correction was performed by subtracting a weighted second-order reflection image.
Images simulated using an x-ray tube with a filtered 120 kVp spectrum clearly showed the position of a phantom containing a 10 µg/ml iodine solution at a radiation dose of 2 mGy. For a monochromatic x-ray source with energy of 60 keV, a reasonable image could be acquired from an iodine concentration of just 1 µg/ml at a dose of 6.8 mGy.
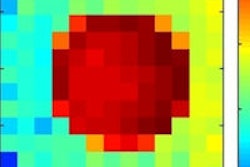
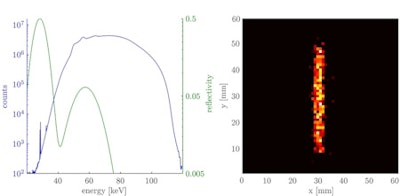 Left: Logarithmic plot of the detected x-ray spectrum averaged over the scanned volume of the 10 µg/ml iodine solution cylinder of the small-animal phantom for an energy bin size of 100 eV. The normal distributions (shown in green) indicate the reflectivity curve of the first and second reflection order of the crystal. Right: Attenuation corrected fluorescence image showing the position of the iodine solution cylinder.
Left: Logarithmic plot of the detected x-ray spectrum averaged over the scanned volume of the 10 µg/ml iodine solution cylinder of the small-animal phantom for an energy bin size of 100 eV. The normal distributions (shown in green) indicate the reflectivity curve of the first and second reflection order of the crystal. Right: Attenuation corrected fluorescence image showing the position of the iodine solution cylinder.The researchers also simulated a human imaging phantom, composing a 30 cm diameter tissue cylinder containing a 2 cm cylinder filled with gold solution. Gold was chosen because iodine fluorescence is strongly attenuated in larger objects, and gold Kα fluorescence has a lower attenuation coefficient in human tissue. For this phantom and a monochromatic x-ray source, the lowest detectable concentration of gold solution was about 100 µg/ml at a radiation dose of 4.2 mGy.
The authors concluded that this novel x-ray fluorescence imaging setup offers the possibility of nonradioactive in vivo molecular imaging of high-Z tracers using conventional x-ray tubes. One initial clinical application of this technique could be within vascular imaging, says Müller, using existing iodine- or gold-nanoparticle-based contrast agents but at far lower concentrations than currently employed. This would enable imaging of patients with renal dysfunction or diabetes.
Another promising application is specific tumor imaging using gold nanoparticles conjugated to bio-molecules. "The successful clinical application of these methods would depend on the outcome of further pre-clinical research on the binding of the gold-nanoparticle based tracers to tumours," Müller told medicalphysicsweb. "Also, the long scan time of the pencil-beam scanning procedure is still problematic. However, this could be overcome by novel x-ray source designs, for example, compact laser-driven x-ray sources. We are currently working on the development of a more complete experimental setup for imaging tracers based on gold-nanoparticles."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.