Researchers from Belgium have crafted a model that discriminates between benign and malignant ovarian tumors, and it also distinguishes between the four types of ovarian malignancy. The model has implications for triage and management decisions, they said.
Ovarian cancer -- the most aggressive gynecological malignancy -- has a relatively poor five-year survival rate at about 40%, so the most important factor for survival becomes stage at diagnosis. Clinicians have tried to develop a screening method, but none currently exists. Many ovarian cancer patients are treated by general surgeons, so an accurate specific diagnosis of adnexal tumors before surgery will improve triage and mean patients receive appropriate treatment.
Recently, the International Ovarian Tumor Analysis (IOTA) group showed that polytomous risk prediction for diagnosing ovarian cancer is feasible, so mathematical models were developed to predict four tumor categories: benign, borderline, primary ovarian cancer, and secondary metastatic cancer. However, there were problems with it, namely the models didn't take into account serum CA-125 levels, nor distinguish between stage I and stage II-IV primary cancer.
Dr. Ben Van Calster, from KU Leuven in Belgium, and 19 colleagues (including the IOTA group) developed a polytomous risk prediction model that can reliably distinguish between benign, borderline, stage I invasive, stage II-IV invasive, and secondary metastatic adnexal tumors based on 5,909 patients.
Their observational diagnostic study prospectively collected clinical and ultrasound data from 24 centers in 10 countries (BMJ, 16 October 2014). Women with an ovarian (including paraovarian and tubal) mass underwent standardized ultrasound exams before surgery.
The Assessment of Different Neoplasias in the Adnexa (ADNEX) model contains three clinical and six ultrasound predictors: age, serum CA-125 level, type of center (oncology centers versus other hospitals), maximum lesion diameter, proportion of solid tissue, and more than 10 ascites.
Using a cutoff of 10% to predict malignancy, the sensitivity was 96.5% and specificity was 71.3% on the validation data, according to the authors. The final ADNEX model is available online at www.iotagroup.org/adnexmodel.
| Area under the receiver operating characteristic curve (AUC) for tumors | |||||
| Benign tumor vs. malignant | Benign vs. borderline | Benign vs. stage I cancer | Benign vs. stage II-IV cancer | Benign vs. secondary metastatic | |
| AUC | 0.94 | 0.85 | 0.92 | 0.99 | 0.95 |
| Stage I cancer vs. borderline | Stage I vs. Stage II-IV | Stage II-IV vs. borderline | Stage I vs. secondary metastatic | ||
| AUCS between malignant subtypes | 0.75 | 0.87 | 0.95 | 0.71 | |
The predicted probability of a specific tumor type is highest for patients with a matching reference standard; for instance, patients with histologically confirmed borderline tumors had the highest probabilities of a borderline malignancy, the researchers wrote.
"Proportion of solid tissue and serum CA-125 level had the strongest independent relations with the outcome, as judged by the test statistic for the model coefficients," they added.
Without using CA-125 as a predictor affected discrimination between stage II-IV cancer and other malignancies, but the ADNEX model still worked.
Here is an example of how the ADNEX model functions when the CA-125 level is known. In a 55-year-old woman, her serum CA-125 level was 42 U/mL. Ultrasound revealed an adnexal mass with more than 10 cystic locules, no papillary projections, no acoustic shadows, ascites, a maximum lesion diameter of 120 mm, and a maximum diameter of the largest solid component of 20 mm.
The ADNEX model gives the following probabilities: 37.4% for borderline tumor, 10.8% for stage I cancer, 8.4% for stage II-IV cancer, and 11.0% for secondary metastatic cancer. The total risk of malignancy is 37.4 + 10.8 + 8.4 + 11.0 = 67.6%. The tumor is most likely a borderline tumor.
If the CA-125 level was unavailable, predicted probabilities would be 25.2% (borderline), 8.3% (stage I), 35.8% (stage II-IV), and 11.5% (metastatic). Baseline probabilities for each type of tumor are 6.3% for borderline tumor, 7.5% for stage I, 14.1% for stage II-IV, and 4.0% for metastatic cancer.
"The ADNEX model has clear potential to optimize management of women with an adnexal tumor," the researchers wrote. "Currently the risk of malignancy index (RMI) is often used to characterize adnexal masses as benign or malignant. However, the index had much poorer performance for discrimination between benign and malignant tumors (AUC 0.88, 67.1% sensitivity, and 90.6% specificity at the typical risk of malignancy index cutoff of 200) than the ADNEX model when tested on our validation data."
Not only does the ADNEX model discriminate between benign and malignant tumors, it also predicts type of malignancy.
"Knowledge of the specific type of adnexal pathology before surgery is highly likely to improve patient triage, and it also makes it possible to optimize treatment. This in turn may reduce morbidity and lead to enhanced survival from different types of ovarian malignancy," they wrote.
One of the drawbacks of ADNEX is that predictions can only be made once the results of blood sample analyses are available.
"We expect that the performance of the ADNEX model will be maintained in the hands of nonexpert ultrasound examiners on condition that the examiners are familiar with the IOTA terms and definitions, and use the IOTA examination and measurement techniques," the researchers wrote. "How the predicted risks from ADNEX should be used clinically must be decided on an individual basis, because patient management depends on many factors," concluded the study authors, adding that ADNEX predictions may form a solid and objective base for optimal management of patients and could be incorporated in national and international clinical guidelines."
The ADNEX model could also be optimized for use as a second stage test if screening for ovarian cancer is introduced into clinical practice, they added.
The researchers plan on regularly updating the ADNEX model coefficients using newly collected data and monitoring the model's performance. They also plan to research patients who are managed conservatively, which is the subject of phase V of the IOTA study.
European Radiology study
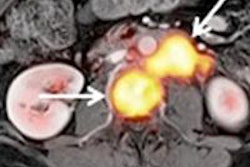
In other news on ovarian cancer, a study published in European Radiology has determined that tracking both tumor-feeding arteries and the ovarian vein using multidetector CT to confirm the origin of large pelvic tumors can distinguish large ovarian tumors from nonovarian tumors.
"In the female pelvis, differential diagnosis between ovarian and nonovarian origin of pelvic tumors is one of the most common problems in imaging," according to Dr. YangKang Li from the department of radiology at Cancer Hospital at the Shantou University Medical College in China. "Establishing the correct diagnosis is important because it directly impacts the staging and treatment of the tumor" (European Radiology, 2 November 2014).
Accurately determining the source organ of these tumors is the first task to obtain correct differential diagnosis on imaging, Li wrote in an email to AuntMinnieEurope.com.
"Misdiagnosis is common for many young radiologists, because they get used to establishing diagnosis and differential diagnosis only through conventional CT images without CT angiography," Li added.
Now, radiologists in Li's department use CT angiography to judge the source organ of a large mass in female pelvis, which postoperative follow-up confirms can fundamentally improve the diagnostic efficiency.
"We are surprised to find that combined application of tumor-feeding arteries and ovarian vein is superior to independently using the ovarian vein or the feeding arteries of the ovary in determining the ovarian origin of a pelvic tumor, and is a useful way to differentiate large ovarian from nonovarian tumors," Li wrote. "This will provide a useful method for radiologists to improve the efficiency of differential diagnosis between large ovarian and nonovarian tumors in clinical imaging practice."
Li plants to carry out prospective studies with a larger sample size in the future.