Cerebrospinal fluid (CSF) is a potential pathway for gadolinium-based contrast agents (GBCAs) into brain tissue, according to a new rat study published online in European Radiology on 10 November. The research may have important implications for humans.
Fluid-attenuated MRI is a useful way to show GBCA distribution with CSF flow, noted lead author Gregor Jost, PhD, from MR and CT Contrast Media Research at Bayer Pharma in Berlin. In their study, GBCA clearance from CSF was almost complete within 24 hours, whereas slightly higher gadolinium concentrations were found in the cerebellum and pons, suggesting delayed excretion from these structures.
In contrast to brain signal hyperintensities, no differences in penetration and distribution into the CSF of healthy rats exist among the marketed GBCAs, confirming that GBCA structure and physicochemical properties have no impact on CSF penetration and distribution, Jost and colleagues explained. All marketed GBCAs cross the blood-CSF barrier to an almost identical extent, but the ability to cross this barrier seems to depend on molecular size as demonstrated by the much lower CSF gadolinium concentration for the macromolecular agent gadomer, which is significantly larger (17 kDa) than the marketed GBCAs (<1 kDa), they stated.
"Dynamic FLAIR [fluid-attenuated inversion recovery] MRI demonstrates a kinetic from the inner CSF spaces to the subarachnoid space and suggests a passive distribution and washout driven by convection and CSF flow," the authors wrote.
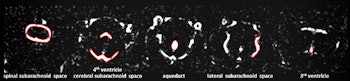
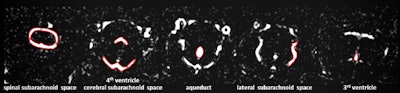
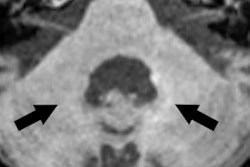
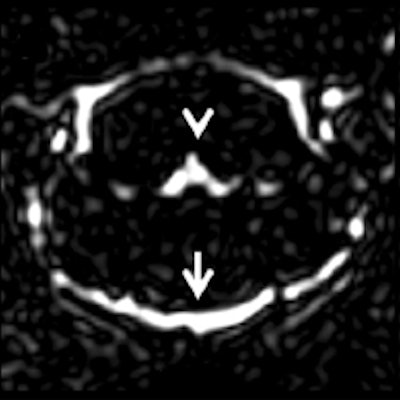
Examples for region of interest (ROI) placement. For the quantitative analysis, ROIs were placed around different CSF spaces using the MR cisternography images for anatomical reference. Images courtesy of Gregor Jost, PhD, reproduced from European Radiology.
They investigated GBCA infiltration and distribution in the CSF in healthy rats using repeated fluid-attenuated MRI up to four hours after high-dose (1.8 mmol/kg) administration of six marketed and one experimental GBCA. Gadolinium measurements in CSF, blood, and brain tissue samples were performed after 24 hours using inductively coupled plasma mass spectrometry.
MRI was performed using a clinical 1.5-tesla machine (Avanto, Siemens Healthineers) and a dedicated two-channel rat head coil. For the evaluation of the fluid space, T2-weighted cisternography (MRC) for anatomical references of the CSF space was used. For MRC, a variable flip angle 3D-TSE sequence (TR = 4,400 ms, TE = 553 ms) with an initial refocusing flip angle of 180° (decreased to 120° for the refocusing echo train) and a turbo factor of 79 were used. The spatial resolution was 0.3 x 0.3 x 0.6 mm (field of view (FOV) = 100 x 48 mm; 30 transversal slices). MRC was followed by pre- and postcontrast heavily T2-weighted FLAIR sequence with identical parameters.
The researchers observed signal enhancement of the CSF spaces in all FLAIR images after GBCA administration. In representative images of the brain at the level of the fourth ventricle, they visualized the cavity fluid spaces by MRC to determine the anatomical location, and they were almost completely attenuated in the baseline FLAIR scan. A clearly visible signal enhancement indicating the presence of GBCA in the fluid spaces was demonstrated by FLAIR imaging. The FLAIR imaging throughout the brain depicted GBCA-induced signal enhancement in all ventricles and the subarachnoid space.
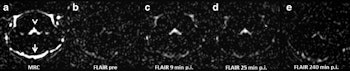
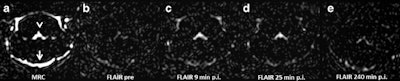
Representative images. The CSF spaces were visualized by MR cisternography, for example the fourth ventricle (arrowhead) and the subarachnoid space (arrow) (a). In the FLAIR images before GBCA injection the respective CSF signal is almost completely attenuated (b). After GBCA administration, a clear signal enhancement of the CSF spaces was found in the FLAIR images up to 240 min postinjection (p.i.) (c-e).
Enhanced MRI signals in the CSF spaces with similar distribution kinetics were observed for all GBCAs, according to Jost et al. They found no substantial differences in the gadolinium concentrations among the marketed GBCAs in the CSF, blood, or brain tissue. After 4.5 hours, the concentration in the CSF was clearly higher than in blood but was almost completely cleared and lower than the brain tissue concentration after 24 hours.
In contrast to the analytical gadolinium quantification, the reduction was not observed with FLAIR MRI as the r1 relaxivity of gadomer is about a factor of five higher than that of the other GBCAs, they continued. After penetrating the blood-CSF barrier, further GBCA distribution within the CSF is driven by diffusion, convection, and CSF flow that are directed through the ventricles to the subarachnoid space of the cortex and spinal cord. The delayed MRI signal increase observed in the subarachnoid space represents this distribution process.
To date, the mechanism of final distribution from the CSF into the brain and specifically to the dentate nucleus and globus pallidus could not be evaluated in this experiment and needs further study, concluded Jost, whose co-authors came from the Institute of Vegetative Physiology at the Charité in Berlin and Nagoya University Graduate School of Medicine in Nagoya, Japan.
Earlier this year, the same lead author published an article about signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents, comparing linear and macrocyclic agents (Investigative Radiology, 2016 Feb;51[2]:83-89). They found increased signal intensity in the deep cerebellar nuclei was up to 24 days after multiple, extended doses of linear GBCAs, but in contrast to clinical reports, the signal enhancement in the globus pallidus was not reproduced, demonstrating the limitations of the study.
The elevated signal intensities remained persistent over the entire observation period, the team reported. In contrast, no changes of signal intensities in either the deep cerebellar nuclei or the globus pallidus were observed for macrocyclic GBCAs, but all GBCAs investigated were able to pass the blood-CSF barrier in rats to a certain, not yet quantified extent.