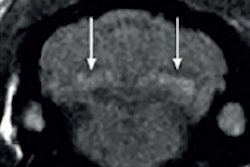
For some months I have been following with interest the uproar about the discovery of gadolinium deposits in brain tissue of some patients after serial MR examinations with nonspecific gadolinium-based contrast agents.
 Dr. Peter Rinck, PhD, is a professor of diagnostic imaging and the president of the Council of the Round Table Foundation (TRTF) and European Magnetic Resonance Forum (EMRF).
Dr. Peter Rinck, PhD, is a professor of diagnostic imaging and the president of the Council of the Round Table Foundation (TRTF) and European Magnetic Resonance Forum (EMRF).The news broke when some radiologists saw high signal intensity stemming from the pituitary gland on T1-weighted MR images -- on nonenhanced images. However, the patients had undergone several contrast-enhanced studies earlier in their life.1-3
It is not clear whether gadolinium found in these cases is still bound to the chelate of a contrast agent, whether it's elemental, or is in another, newly formed compound; the latter seems most likely. There is strong evidence that the deposit of gadolinium can be traced back to the linear agents gadodiamide (Omniscan) and gadopentetate dimeglumine (Magnevist).4
As early as 1988, at one of the first big and independent meetings on contrast agents developed for MRI, Michael F. Tweedle pointed out that Magnevist could become unstable in vivo and release free gadolinium, whereas macrocyclic compounds such as Gd-DO3A (ProHance) and Gd-DOTA (Dotarem) remain stable.5
At the same meeting, researchers from the company producing Gd-DOTA presented similar results and stressed the importance of macrocyclic compounds "to minimize the in vivo dissociation process and avoid potential biological disturbances produced by the presence of free species."6
What happens to free gadolinium?
During the early preclinical development of Magnevist, Weinmann and his co-authors7 compared the pharmacokinetics of Gd-DTPA and of gadolinium trichloride in rats. They found that 80% of Gd-DTPA was excreted from the organism in urine within three hours, and after seven days, 90% of the dose was recovered in urine and another 7% in the feces. Less than 0.3% was found in the body, with 0.08% detected in the liver and 0.1% in the kidneys.
In the case of free gadolinium (i.e., gadolinium trichloride), only 2% was excreted after seven days. The rest remained all over the body, mostly in the liver and in the spleen, one-sixth elsewhere. The conclusion was that chelates such as DTPA can be extremely effective to remove the highly toxic, but diagnostically very helpful, gadolinium from the body.
However, if the chelate doesn't work properly, patients might be at risk.
The amount of gadolinium one needs to enhance contrast in pathologies on T1-weighted MR images is minimal -- particularly compared with the amounts of iodine needed for x-ray contrast agents. Nearly everybody believed what they were told: There were hardly any acute side effects; in general, "gado" is safer than iodinated x-ray contrast agents, which is true. As always with drugs, the dose and the galenics make the distinction between poison and remedy.
My first encounter with what would become Magnevist was more than 30 years ago. At that time, independent researchers at universities were more interested in the performance of the agents than in their safety, in particular because the safety was guaranteed by the manufacturers. We trusted the characteristics of, e.g., the gadolinium-DTPA complex that were presented to us. The challenge was how to apply the compound within the given limits -- which was worked out step by step.
Soon the new compounds were used for all conceivable kinds of examinations. One outstanding example is the follow-up of treatment of multiple sclerosis (MS) patients -- in some instances MS patients underwent contrast-enhanced studies once a month for two or three years. The general attitude for all medical or not-so-medical indications was: "Gado is a dye and can be used repetitively as often as possible."
Recommended dose, best enhancement
On some of the first MR images in animals and in humans, parts of the body were highlighted after the injection of Gd-DTPA, but other areas turned black. The pituitary gland became bright like a streetlight, the bladder dark. The question was: Why? The answer was found in the correct dose to be injected.
The level of 0.1 mmol/kg body weight soon became the accepted standard. This dose provides excellent enhancement at low and medium magnetic fields. The reason is easily visible on the animated simulation shown here.
A dose increase beyond the recommended dose may lead to loss of contrast. This is because a T2 shortening remains and can take over primary influence upon image contrast.
Parallel to the nonspecific gadolinium agents, angiographic blood-pool agents were being developed because nonenhanced imaging technologies did not fulfill the requirements for high-resolution angiography. The manufacturers anticipated a substantial market because contrast-enhanced MR angiography was to cut a big slice out of the conventional and CT angiography cake. Yet, the development of the blood-pool agents was slow and plagued by setbacks.
Then, suddenly a group of doctors proposed the use of the existing nonspecific agents together with special patented techniques and hardware to perform MR angiography. They all underlined that high-dose gadolinium chelates (up to 0.3 mmol/ kg) were significantly less nephrotoxic than iodinated contrast agents.8 In a textbook of contrast-enhanced MR angiography, Martin R. Prince, Thomas M. Grist, and Jörg F. Debatin stated in 2003:
"From an image quality point of view, generally the more contrast the better ... Gadolinium compounds have no clinically detectable nephrotoxicity. They can be used safely at the maximum dose in patients with renal failure."9
Soon there were patent agreements with numerous pharmaceutical and hardware companies.10
Origins of nephrogenic systemic fibrosis
Then, early in 2006, there was an outbreak of a new disease in Austria: Nephrogenic fibrosing dermopathy, later called nephrogenic systemic fibrosis (NSF). Single cases had been described earlier, but not attributed to gadolinium:
"NFD was unknown before March 1997 and some authors suggest that the sudden occurrence of the disease in the last eight years makes it likely that a new agent or technique of examination causes NFD/NSF."11
Among others, Martin R. Prince reacted with a paper describing 15 patients who developed NSF after high-dose gadolinium-enhanced MR imaging compared with zero patients with the standard dose. The conclusion of the paper included the following sentence:12
"We recommend using no more than a standard dose of GBCA (0.1 mmol/ kg)."
Shortly after the publication of this article, a letter reached the editor of Radiology and was published. The author stated:13
"The finding that all patients who developed NSF had received a high dose of a gadolinium-based contrast agent (GBCA), but none of the 74,124 who had received a standard dose (0.1 mmol per kilogram of body weight) developed NSF, irrespective of renal function, is of particular interest ...
"Perhaps it is linked to the development of techniques that require the use of higher doses of GBCA, such as contrast agent-enhanced magnetic resonance (MR) angiography. For better visualization, especially of distal and small vessels, double or triple doses of GBCA are often advocated ...
"This temporal coincidence may only be incidental, but it is nonetheless suggestive and may help to explain the somewhat mysterious timing of the appearance of NSF."
Admittedly, nowadays one finds the following warning in the package insert: "Do not exceed the recommended dose ... and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration." Yet, the positions of the U.S. Food and Drug Administration (FDA) and their European counterparts leave conflicting impressions, to put it politely.
Now we are confronted with a new challenge: gadolinium deposits in the brain.
Already the vultures are circling and the ambulance chasers are flooding the Internet with their websites: the lawyers are coming in great number. The motto is: "Are you gadolinium-toxic? If yes, contact us." They are rather clever and business-minded. And they will soon find the arguments with which they will milk the manufacturers, the radiologists -- and, most of all, their clients. The arguments will most likely go like this:
"Lack of background knowledge of the radiologists and their incredible trust in pharmaceutical companies; the almighty dollar sign; and use of an inappropriate and unsafe drug, too high a dose, too many serial studies, too close a study after another, examinations too often without proper indication; off-label use."
Debacle is as good a word as any to describe what has happened here; and many of the parties involved try to sweep the problem under the rug: manufacturers, radiologists, and supervising administrations.
What is the clinical significance of gadolinium deposits in the brain and elsewhere? Nobody really knows. However, it doesn't belong there. NSF is a new iatrogenic disease. The (unlimited?) storage of gadolinium in the human body is but a continuation of this disease.
The recent events coincide with descriptions of the sudden appearance of gadolinium as anthropogenic contamination in tap water.14 The cause is the use of MR contrast agents; gadolinium cannot be removed by water treatment plants.
Important Note: Contrast-enhanced MR examinations have life-saving benefits. I fully support the intravenous application of gadolinium-based contrast agents for diagnostic purposes. However, please apply macrocyclic contrast agents that are safe; even they should be used only if a clear diagnostic advantage for the patient can be expected.
Dr. Peter Rinck, PhD, is a professor of diagnostic imaging and the president of the Council of the Round Table Foundation (TRTF) and European Magnetic Resonance Forum (EMRF).
References
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841.
- Kanda T, Osawa M, Oba H, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: Association with linear versus macrocyclic gadolinium chelate administration. Radiology. 2015;275(3):803-809.
- Radbruch A, Weberling LD, Pascal J, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015; 275(3):783-791.
- Rinck PA. Radiologists meet with heavy collateral damage. Rinckside. 2008;19(3):7-10.
- Tweedle MF. Work in progress toward nonionic macrocyclic gadolinium (III) complexes. In: Rinck PA (ed). Contrast and contrast agents in magnetic resonance imaging. Proceedings of Contrast and Contrast Agents in Magnetic Resonance Imaging -- A Special Topic Seminar; Trondheim, Norway; 12-13 September 1988. Trondheim and Mons: The European Workshop on Magnetic Resonance in Medicine (EMRF). 1989. 65-73.
- Meyer D, Schaefer M, Doucet D. Physico-chemical properties of the macrocyclic chelate Gadolinium-DOTA. In: Rinck PA (ed). Contrast and contrast agents in magnetic resonance imaging. Proceedings of Contrast and Contrast Agents in Magnetic Resonance Imaging -- A Special Topic Seminar; Trondheim, Norway; 12-13 September 1988. Trondheim and Mons: The European Workshop on Magnetic Resonance in Medicine (EMRF). 1989. 33-43.
- Weinmann HJ, Brasch RC, Press W-R, Wesbey GE. Characteristics of Gadolinium-DTPA complex: A potential NMR contrast agent. AJR. 1984;142(3):619-624.
- Prince MR, Arnoldus C, Frisoli JK. Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imaging. 1996;6(1):162-166.
- Prince MR, Grist TM, Debatin JF. 3D contrast MR angiography. Berlin, New York: Springer Publishers. 3rd ed. 2003;22-23.
- Ersoy H, Zhang HL, Prince MR. Peripheral MR Angiography. J Cardiovasc Magn Reson. 2006;8(3):517-528.
- Grobner T. Gadolinium -- a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006; 21:1104-1108. Important erratum: Nephrol Dial Transplant. 2006;21:1745.
- Prince MR, Zhang H, Morris M et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008; 248(3):807-816.
- Gossner J. Letter to the editor [concerning Prince MR, et al. Radiology. 2008;248(3):807-816]; and response by Prince MR, et al. Radiology. 2009;251(2):612-613.
- Tepe N, Romero M, Bau M. High-technology metals as emerging contaminants: Strong increase of anthropogenic gadolinium levels in tap water of Berlin, Germany, from 2009 to 2012. Applied Geochemistry. 2014;45(32):191-197.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.