Radiopharmaceuticals can interact with drugs and other agents with sometimes detrimental consequences. What if there were a downloadable database that categorized such interactions? Spanish researchers have created one, calling it Datinrad.
Radiopharmaceuticals usually have no pharmacologic effects because they're used in trace quantities, so they don't follow the typical dose-response relationship found in conventional drugs. However, because radiolabeled particles present some potential mechanical issues, such as the embolic effect created by technetium-99m macroaggregated albumin and yttrium-90 resin particles or beads, a sufficient number of particles need to be administered to avoid a nonuniform spatial distribution of radioactivity in lung regions, for instance.
 Jesús Luis Gómez Perales, PhD, plans to regularly update the radiopharmaceutical database Datinrad he helped to create.
Jesús Luis Gómez Perales, PhD, plans to regularly update the radiopharmaceutical database Datinrad he helped to create.On the other hand, an excess of radiopharmaceutical particles can produce acute toxicity, especially in patients with severe pulmonary hypertension. Thus, it's important to determine the ideal number of particles for a lung scan, wrote a researcher team led by Jesús Luis Gómez Perales, PhD, from the Nuclear Medicine Service at Puerta del Mar University Hospital in Cádiz, Spain, in an article published July 15 in the Journal of Nuclear Medicine Technology. Co-author on the study is Ana Agudo Martínez, PhD, from the Nuclear Medicine Service at Virgen Macarena University Hospital in Seville, Spain.
"The biodistribution or pharmacokinetics of radiopharmaceuticals may be altered by a variety of drugs, disease states, and surgical procedures, which can have a significant clinical impact on safety, scan interpretation, and diagnostic imaging accuracy," the authors stated. "In their most extreme manifestations, unanticipated imaging results may even compromise the utility or accuracy of nuclear medicine studies."
The researchers acknowledge that the occurrence of adverse events in nuclear medicine has been rare, but they may become more frequent in the future because of the administration of contrast agents in hybrid imaging and the increasing use of therapeutic radionuclides that have a significant risk of subacute and chronic toxicity. Therefore, greater diligence is required in reporting adverse events, an effort that would be aided by having more easily accessible reporting systems.
 Ana Agudo Martínez, PhD, is a co-creator of Datinrad, a radiopharmaceutical database.
Ana Agudo Martínez, PhD, is a co-creator of Datinrad, a radiopharmaceutical database."Information on drug interactions with radiopharmaceuticals is becoming increasingly abundant -- so much so that the nuclear medicine staff can feel overwhelmed," Perales and Martínez wrote. "The challenge, therefore, is to keep all this information organized and accessible."
Also, there is no single system for reporting adverse events associated with radiopharmaceuticals, and different countries have different systems.
The authors developed Datinrad, a database application that allows entry, storage, and retrieval of radiopharmaceutical interactions with drugs or other agents and adverse effects of radiopharmaceuticals.
"Such an application can serve as a means to collect published information -- both previous and new -- and as an easy-to-use reference tool for nuclear medicine specialists in their daily clinical practice," they wrote.
Datinrad was developed and compiled with a commercially available data management system and programming language. All data entered into the database came from the scientific literature and were accompanied by their bibliographic references, the researchers wrote. To date, the database contains 275 drug interactions and 44 records of adverse reactions to radiopharmaceuticals.
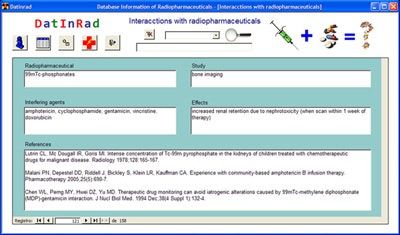
Datinrad can be downloaded for free to a PC from Radiopharmacy.net, a website that the group maintains that also includes other nuclear medicine applications. The database runs on either desktop or laptop computers; the team is also developing an Android app for smartphones, Perales said.
Users can choose between two options for updating Datinrad: their own copy, which allow them to add, delete, or modify records; or a downloaded version the authors will update and make available every year or so as they add new data appearing in the literature. The two options are not mutually exclusive: Users who choose to personalize their copy can also download the updated database.
In an email interview with AuntMinnieEurope.com, Perales stated that since the study has been published in the Journal of Nuclear Medicine Technology, there have been 259 downloads of Datinrad and counting. The next step for Datinrad is to keep it updated and develop the Android version for smartphones, he added.
Collecting data on drug interactions with radiopharmaceuticals and adverse reactions to radiopharmaceuticals, as well as providing all nuclear medicine staff with access to them, may help decrease the incidence of adverse reactions to radiopharmaceuticals and prevent misdiagnoses, the researchers concluded in their study.
"To this end, the database software application Datinrad might play an important role and would be particularly welcomed as a quick guide for routine daily use by nuclear medicine staff in hospitals," they wrote.