In a new study published in the May issue of the Journal of Nuclear Medicine, Dutch researchers are reporting success with whole-body PET/CT as a way to determine the efficacy of bevacizumab treatment for pediatric brain tumors known as diffuse intrinsic pontine glioma (DIPG).
The findings indicate adequate uptake of bevacizumab labeled with zirconium-89 (Zr-89) within the tumors, and the results could help predict the drug's therapeutic potential (JNM, May 2017, Vol. 58:5, pp. 711-716).
Bevacizumab is an antiangiogenic monoclonal antibody designed to bind to tumors. It is marketed as Avastin by Genentech.
Children with DIPG have a poor prognosis, with fewer than 10% of patients surviving two years from diagnosis, the authors noted. The tumors are resistant to therapy, which may not reach the tumor due to its location within the brainstem.
In this study, researchers from VU University Medical Center in Amsterdam measured the tumor uptake of Zr-89-labeled bevacizumab with PET, evaluating the safety of the procedure and determining the optimal timing of imaging.
Two weeks after completing radiotherapy, seven patients (age, 6-17 years) underwent whole-body PET/CT scans at 1, 72, and 144 hours after injection. The optimal moment of scanning was found to be 144 hours. The patients also had gadolinium-enhanced MRI scans.
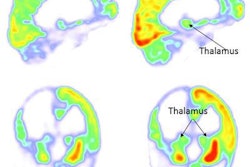
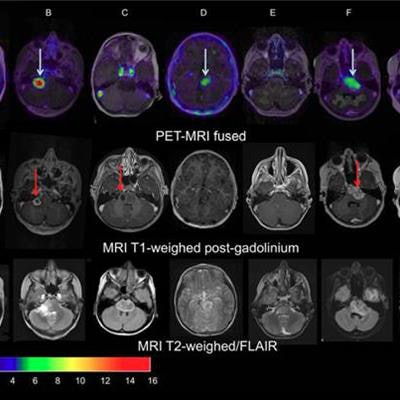
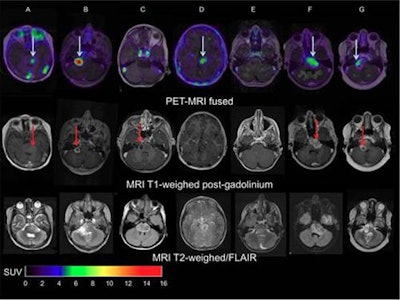 Images of pediatric patients with DIPG show results from Zr-89-bevacizumab PET fused with T1 gadolinium-weighted MRI per patient (top row); T1 gadolinium-weighted MRI (middle row); and T2-weighted/fluid-attenuated inversion recovery (FLAIR) MRI (bottom row). Five tumors show variable uptake of Zr-89 bevacizumab (white arrows), with both PET-negative and PET-positive areas within each tumor. Image courtesy of Sophie Veldhuijzen van Zanten and Marc Jansen, VU University Medical Center.
Images of pediatric patients with DIPG show results from Zr-89-bevacizumab PET fused with T1 gadolinium-weighted MRI per patient (top row); T1 gadolinium-weighted MRI (middle row); and T2-weighted/fluid-attenuated inversion recovery (FLAIR) MRI (bottom row). Five tumors show variable uptake of Zr-89 bevacizumab (white arrows), with both PET-negative and PET-positive areas within each tumor. Image courtesy of Sophie Veldhuijzen van Zanten and Marc Jansen, VU University Medical Center.The researchers, led by Dr. Marc Jansen, found "considerable heterogeneity" in the uptake of Zr-89-labeled bevacizumab within the young patients' tumors. Based on the results, the approach could help predict how well treatment will attack the cancer and to what degree therapies may be toxic, they concluded.